["f3af8dbe37a29fdc17156180c690ea44580a9437"]
Gynecomastia
gynecomastia
pseudogynecomastia
lipomastia
5395
5395
Chapter reads
2
2
Chapter likes
7/10
Evidence score
11
11
Images included
04
4
Videos included
01
Introduction
Introduction
Gynecomastia means unilateral or bilateral benign enlargement of the male breast. It can lead to female appearance and consecutively to severe strain of those affected.
The true gynecomastia with actual hypertrophy of glandular tissue is differentiated from the so-called pseudogynecomastia with mainly fatty tissue growth typically associated with adiposity.
Both types can result in considerable psychological stress and disturbance of the self-confidence. A retrospective single center analyses of 35 patients revealed that gynecomastia in adolescents presents a psychological threat to normal self-esteem as well as sexual identity1.
Hence, gynecomastia represents a serious disorder that needs to be treated appropriately.
The true gynecomastia with actual hypertrophy of glandular tissue is differentiated from the so-called pseudogynecomastia with mainly fatty tissue growth typically associated with adiposity.
Both types can result in considerable psychological stress and disturbance of the self-confidence. A retrospective single center analyses of 35 patients revealed that gynecomastia in adolescents presents a psychological threat to normal self-esteem as well as sexual identity1.
Hence, gynecomastia represents a serious disorder that needs to be treated appropriately.
02
Anatomy of the male breast
Anatomy of the male breast
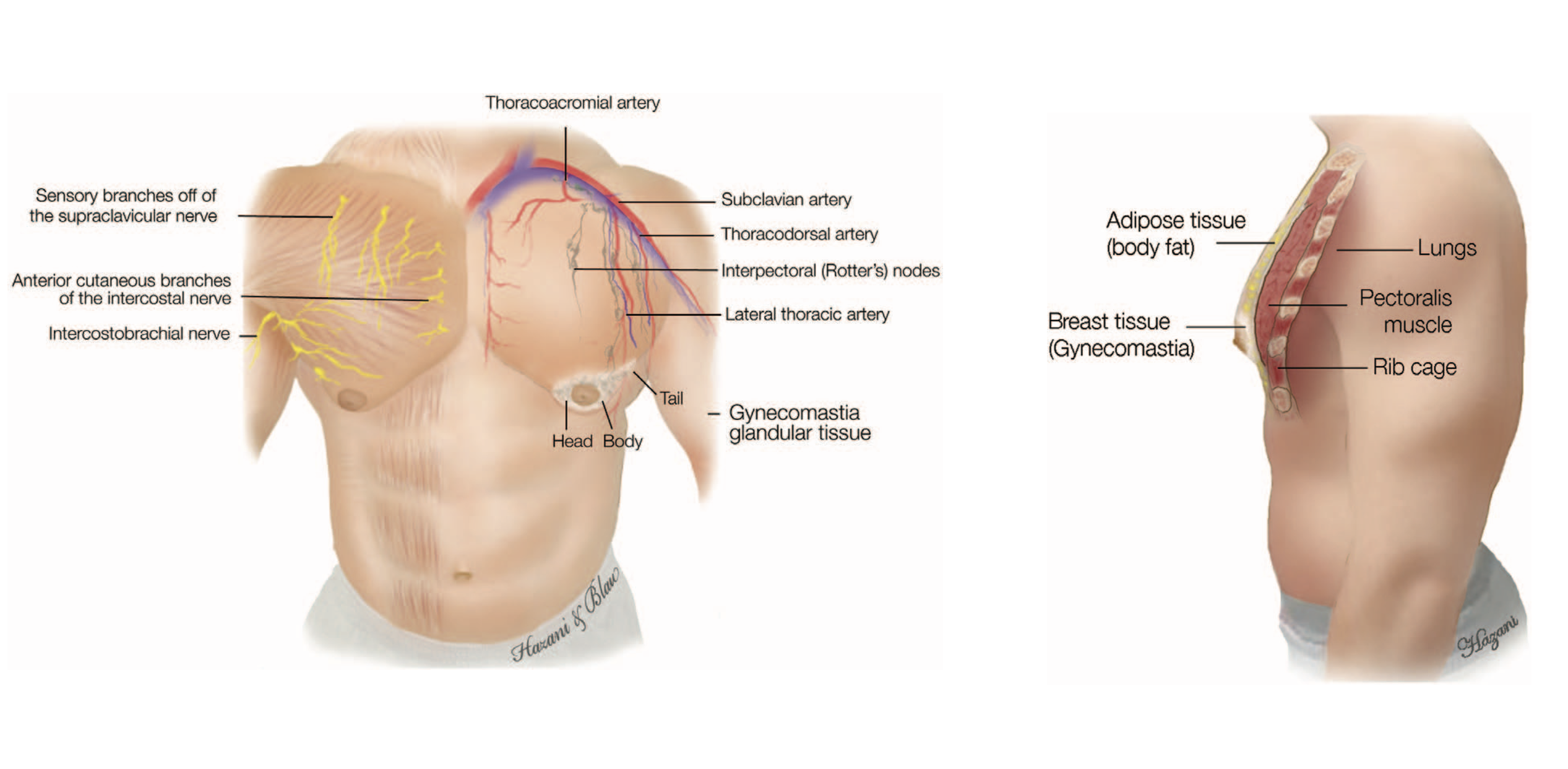
Anatomy male breast
Topography of the ideal male breast
Flat shape accentuating the pectoralis major muscle
Extends from the 2nd to 6th anterior rib
Medial border: sternum
Lateral border: mid-axillary line
NAC (nipple areola complex):
smaller compared to female and slightly oval (average NAC diameter: 26 x 20 mm) [2-4]
slightly cephalad to the caudal border of the pectoralis major muscle [2, 5]
intercostal space between 4th and 5th rib [4-6]
the ideal NAC position is rather depending on BMI and different body shapes than on average body measurements [7] – e.g. with increasing BMI the NAC is located more lateral [8, 9].
--> Respecting the thorax circumference the algorithm of Beer et al. [2] seems most reasonable for the calculation of the idela NAC position:
--> Respecting the thorax circumference the algorithm of Beer et al. [2] seems most reasonable for the calculation of the idela NAC position:
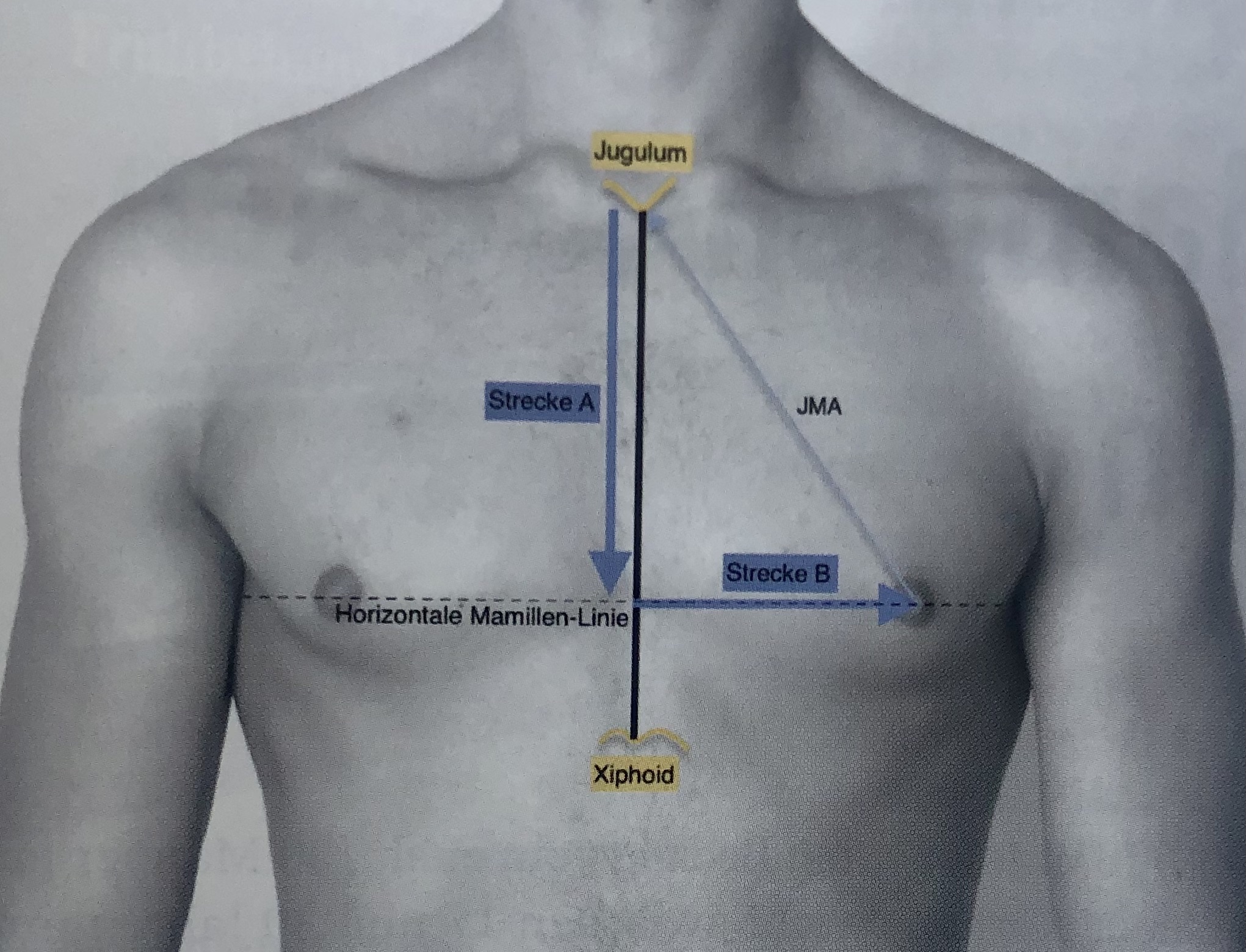
Ideal position of the male nipple-areola complex.- Modified from Beer et. al., by Given et al. Plastische Chirurgie, 16. Jahrgang Heft 2/2016
Distance A = 1.2 + (.28 x length of sternum) + (0.1 x thorax circumference) [cm]
Distance B = 2.4 + (0.09 x thorax circumference) [cm]
modified from Beet et al. - PRS 2001 Dec;108(7):1947-52; discussion 1953. doi: 10.1097/00006534-200112000-00015. [2]
Distance B = 2.4 + (0.09 x thorax circumference) [cm]
modified from Beet et al. - PRS 2001 Dec;108(7):1947-52; discussion 1953. doi: 10.1097/00006534-200112000-00015. [2]
Vascular supply
Arterial and venous supply follow the same pattern. Breast parenchyma and skin are supplied by:
perforating branches of internal mammary artery
lateral thoracic artery
thoracodorsal artery
intercostal perforators
Textthoracoacromial artery
Innervation
Breast innervation is provided by the supraclavicular nerves and by the lateral and median branches of the intercostal nerves - similar to the female breast:
The supraclavicular nerves are said to innervate the upper part of the breast excluding the gland [10]
Branches of the 6th intercostal nerve supply the lower part of the breast, but there seems no direct branch to the NAC [11]
A deep branch from the anterior division of the 4th lateral cutaneous branch is reported to innervate the NAC by taking a course along the superficial fascia and building a plexus underneath the areola [11]
Composition of the male breast tissue
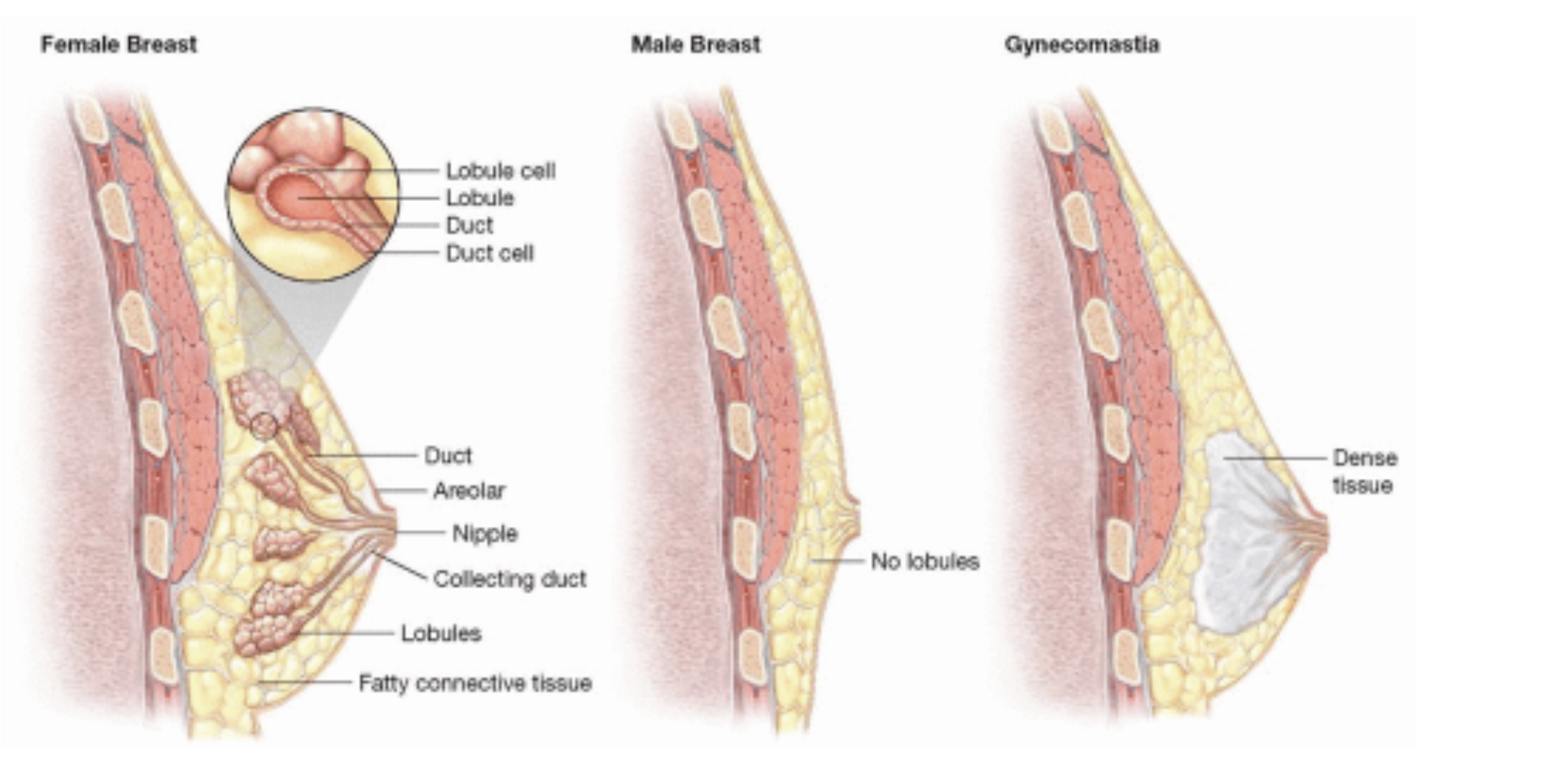
Female breast - Regular male breast - Gynecomastia
The male breast without gynecomastia mainly consists of fatty tissue with sporadic appearance of ducts and stroma [12].
Bannayan et al. histologically classfied the true gynecomastia into florid (ductal hyperplasia and proliferation), fibrous (more stromal fibrosis and fewer ducts) and intermediate gynecomastia [13].
In Contrast to female breast tissue lobules are generally absent [12].
Beside the lipomatous gynecomastia (pseudo-gynecomastia) which is more frequent in older patients, the histological type of the true gynecomastia also seems to be associated with age. Investigations by Fricke et al. showed that the rate of florid gynecomastia increased with the age of patients, while fibrous gynecomastia was more common in adolescents and young adults. The florid one seems to show a higher recurrence rate compared to the fibrous one [14].
Blau et al. demonstrated in their investigations that the male breast gland is composed of a head, body and tail. The head is semicircular medial to the areola, the body is located beneath the areola and the tail goes along to the humeral insertion of the pectoralis major muscle [3].
Bannayan et al. histologically classfied the true gynecomastia into florid (ductal hyperplasia and proliferation), fibrous (more stromal fibrosis and fewer ducts) and intermediate gynecomastia [13].
In Contrast to female breast tissue lobules are generally absent [12].
Beside the lipomatous gynecomastia (pseudo-gynecomastia) which is more frequent in older patients, the histological type of the true gynecomastia also seems to be associated with age. Investigations by Fricke et al. showed that the rate of florid gynecomastia increased with the age of patients, while fibrous gynecomastia was more common in adolescents and young adults. The florid one seems to show a higher recurrence rate compared to the fibrous one [14].
Blau et al. demonstrated in their investigations that the male breast gland is composed of a head, body and tail. The head is semicircular medial to the areola, the body is located beneath the areola and the tail goes along to the humeral insertion of the pectoralis major muscle [3].
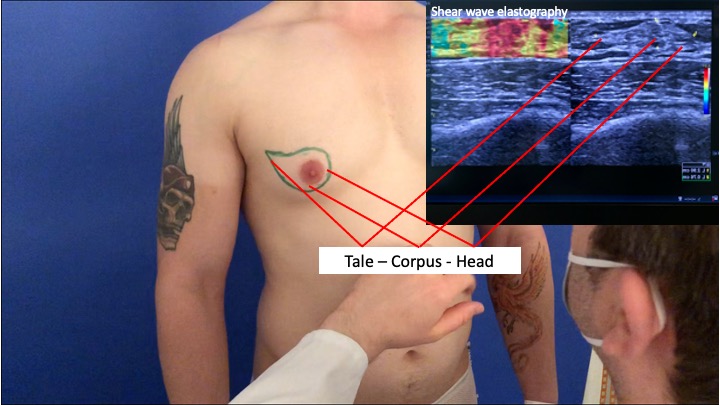
Shear wave elastography of the male gland
Body, head, tail of male gland
03
Etiology and Pathology
Etiology and Pathology
The true gynecomastia is differentiated from the so-called pseudo-gynecomastia (hypertrophy of fatty tissue) and contains true glandular tissue including ducts and stroma (no lobules). It is the most common breast alteration in males and occurs particularly during infancy, puberty and old age [15, 16].
The true gynecomastia is often related to a relative or absolute estrogen excess and/or a decrease of circulating androgen respectively a defect in androgen receptors.
Prevalence rates as reported by Johnson et al.:
The true gynecomastia is often related to a relative or absolute estrogen excess and/or a decrease of circulating androgen respectively a defect in androgen receptors.
Prevalence rates as reported by Johnson et al.:
Newborns: 60–90 %
Adolescents: 50–60 %
Men between 50 and 69 years: 70 %
The physiologic gynecomastia (25%) as part of normal physiologic changes is differentiated from the non-physiologic gynecomastia (50%) which is induced by pathological causes or drugs (see table below). The cause of the idiopathic gynecomastia mostly remains unclear (25%) [17]. Gynecomastia particularly in newborns and adolescents disappears spontaneously within months or years [18].
The histological appearance is reported to change with duration of symptoms[13]:
The histological appearance is reported to change with duration of symptoms[13]:
Florid: <4 months
Intermediate: 4-12 months
Fibrous: >12 months
Spontaneous regression of fibrous gynecomastia is regarded as unlikely. Thus, surgical treatment of gynecomastia is recommended after >12 months of persistence [18, 19].
Physiological gynecomastia
Transient neonatal gynecomastia
60-90 % of neonates due to transplacental transfer of maternal oestrogens
Transient pubertal gynaecomastia
50-60 % of adolescents, between ages 12 -14 to 19-21, typically lasting 6-12 months, spontaneous regression 90% due to disparity of estrogen/testosterone levels and increased sensitivity to estrogen 5-8.
Age related gynaecomastia
up to 70 % of men ages over 65, likely related to reduced testosterone levels and testicular involution 2, 9.
Pathological causes of gynecomastia
Gonadal failure
Primary hypogonadism due to trauma, chemotherapy, inflammatory damage, chromosomal aberration as Klinefelter Syndrome. In Klinefelter’s syndrome the reported incidence of gynecomastia syndrome ranges between 56% and 88% 10.Secondary hypogonadism due to hyperprolactinaemia or hypothalamic-pituitary axis, leading to lack of stimulation (pituitary adenomas).
Thyroid dysfunction
Hyperthyroidism leads to increase of sex hormone binding globulin, reducing free testosterone. Cause of gynecomastia in 10-40% of cases [27]. Early regulation to euthyroid state can resolve gynecomastia.
Renal insufficiency
Caused by suppression of testosterone production and possibly by direct testicular damage due to uremia[28]
Hormone secreting tumours
Estrogen secreting (Sertoli cell tumor, Leydig cell tumor), human chorionic gonadotrophin secreting tumors – in 7-11% of testicular tumors gynecomastia is the only symptom[29, 30], Testicular tumors are present in 3% of men with gynecomastia[31, 32].
Obesity
More often associated to pseudo-gynecomastia but also results in high levels of leptin and aromatase activity, increasing estrogen levels[33]
Other conditions
Ulcerative colitis; Cystic fibrosis; Refeeding syndrome after a prolonged period of malnutrition; Testicular infiltration-tuberculosis haemochromatosis
Drugs known to induce gynecomastia [34-37]
Antiandrogens
bicalutamide, flutamide, finasteride, dutasteride (AA)
Antiretrovirals
protease inhibitors (saquinavir, indinavir, nelfinavir, ritonavir, lopinavir), reverse transcriptase inhibitors (stavudine, zidovudine, lamivudine) (UM), efavirenz (UM)
Antihypertensives
calcium channel blockers (amlodipine, diltiazem, felodipine, nifedipine, verapamil) (UM)
spironolactone (AA) -(as one of the most frequent causes)
spironolactone (AA) -(as one of the most frequent causes)
Environmental exposures
phenothrin (antiparasitical)
Exogenous hormones
oestrogens (EP), prednisone (male teenagers), human chorionic gonadotrophin (E)
Gastrointestinal drugs
H2 histamine receptor blockers (cimetidine) (AA), proton pump inhibitors (eg, omeprazole) (AA)
Analgesics
opioid drugs (RA)
Antifungals
ketoconazole (prolonged oral use) (AA)
Antipsychotics (first generation)
haloperidol (IP), olanzapine, paliperidone (high doses), risperidone (high doses), ziprasidone
Chemotherapy drugs
methotrexate, alkylating agents—eg, cyclophosphamide, melphalan (AA); carmustine, etoposide, cytarabine, bleomycin, cisplatin (AA), vincristine (AA), procarbazine
Exogenous hormones
androgens (misuse by athletes) (EP)
Cardiovascular drugs
phytoestrogens (soya based products, high quantity) (EP)
Recreational/illicit substances
marijuana, amphetamines (UM), heroin (UM), methadone (UM), alcohol
Herbals
lavender, tea tree oil, dong quai (female ginseng), Tribulus terrestris, soy protein (300 mg/day), Urtica dioica (common nettle)
AA=antiandrogenic; RA=reduced androgens; E=oestrogenic; IAM=increased androgen metabolism; ISHBG=increased concentration of sex hormone binding globulin; IP=increased prolactin; UM=unknown mechanism
Overview modified from Paul Thiruchelvam et al. "Gynecomastia" - Clinical updates - BMJ 2016;354:i4833 doi: 10.1136/bmj.i4833
04
Male breast cancer
Male breast cancer
Male breast cancer is a very rare disease and represents less than 1-2% of all malignancies in men and less than 1% of all breast cancers [25]:
Lifetime risk: 1/1000 [25]
Median age at diagnosis in US: 62.4 years (vs. 58.2 in women) [25]
> 35 years the incidence increases sharply and does not show a midlife decline as observed in women [26]
Particular patient groups with high risk factors show increased incidences [25,26]:
Mutation in BRCA-2 gene increases the incidence by 80-100 times (Lifetime risk: 6.9%)
Klinefelter Syndrome increases the incidence by 20-50 times (low androgen and high gonadotropin levels due to atrophic testes)
Positive family history of breast cancer (33% of all male breast cancer patients within families with positive family history for breast or ovarian cancer )
Due to the low incidence of male breast cancer in patients without high risk factors it seems reasonable to treat gynecomastia using liposuction or surgical excision. In those with high risk factors as BRCA-2 mutation or Klinefelter syndrome excisional techniques are preferred [27].
05
Diagnosis
Diagnosis
Anamnesis
Onset time
Course of development
Drug history
History of associated co-diseases
Pharmaceuticals
Alcohol
Drugs
Family history with regards to cancer
Exposition to radiation
Examination
Body mass index
Bilateral comparative breast palpation including the axilla 3:
Supine position with arms resting behind head
Palpation with regards to painfulness, size and resistance
Size evaluation and documentation for follow up
> 2 cm in a concentric glandular mass is most consistent with true gynecomastia
< 2cm is defined as breast tissue which increases with age and adiposity
Suspicious breast masses (unilateral, decentral, painless, peau d'orange, rapid growth) -> referral to breast specialist
Testicular examination with respect to hypogonadismus or testicular masses
Consider Klinefelter syndrome
Technical Investigations
If breast tissue increased gradually and physiological gynecomastia seems reasonable no further investigations are required. For all other cases where no underlying cause can be determined further investigations are recommended.
Blood tests [40]
Standard tests: testosterone, estradiol, luteinizing hormone (LH), follicle-stimulating hormone (FSH), prolactin, sex hormone-binding globulin (SHBG), α-fetoprotein, ß-hCG, GOT, creatinine
Thyroid function: TSH, T3, T4
Suspicion of Klinefelter: chromosomal analysis
Imaging [40]
Imaging of the male breast:
Standard: Ultrasound is generally recommended also with regards to the differentiation between true gynecomastia and lipomastia.
In line with US guidelines further imaging investigation is only required where physical examination is unclear or suspicious 12. Therefore Mammography is the method of choice 13. Due to the fact that the incidence of gynecomastia associated breast cancer is reported with 0-2.5% mammography is rarely required 14.
Further Imaging:
Standard: Ultrasound is generally recommended also with regards to the differentiation between true gynecomastia and lipomastia.
In line with US guidelines further imaging investigation is only required where physical examination is unclear or suspicious 12. Therefore Mammography is the method of choice 13. Due to the fact that the incidence of gynecomastia associated breast cancer is reported with 0-2.5% mammography is rarely required 14.
Further Imaging:
Standard: testicular ultrasound is always recommended
Suspicion of a tumor of the adrenal glands (estradiol ↑, LH↓) : abdominal ultrasound, Ct-scan,12.
Suspicion of pituitary adenoma (prolactin↑, LH+FSH ↓): MRI
06
Clinical and Operative Algorithm
Clinical and operative Algorithm
Clinical Algorithm
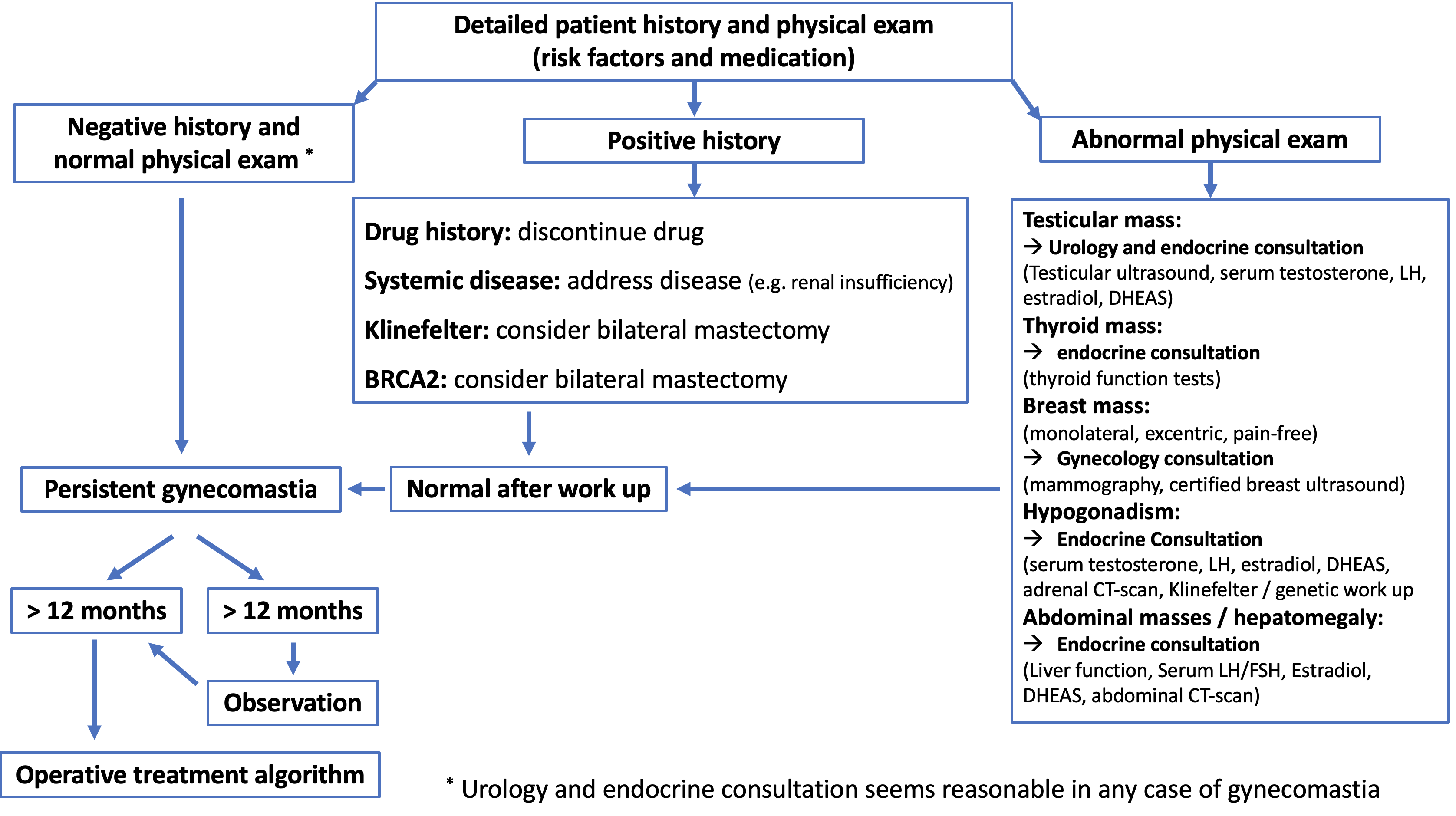
Clinical algorithm (modified from: Rohrich, R.J., et al., Plast Reconstr Surg, 2003. 111(2): p. 909-23; discussion 924-5.)
Operative Treatment Algorithm
Authors preferred operative management
07
Classifications
Classifications
Clinical Classifications
Classification History
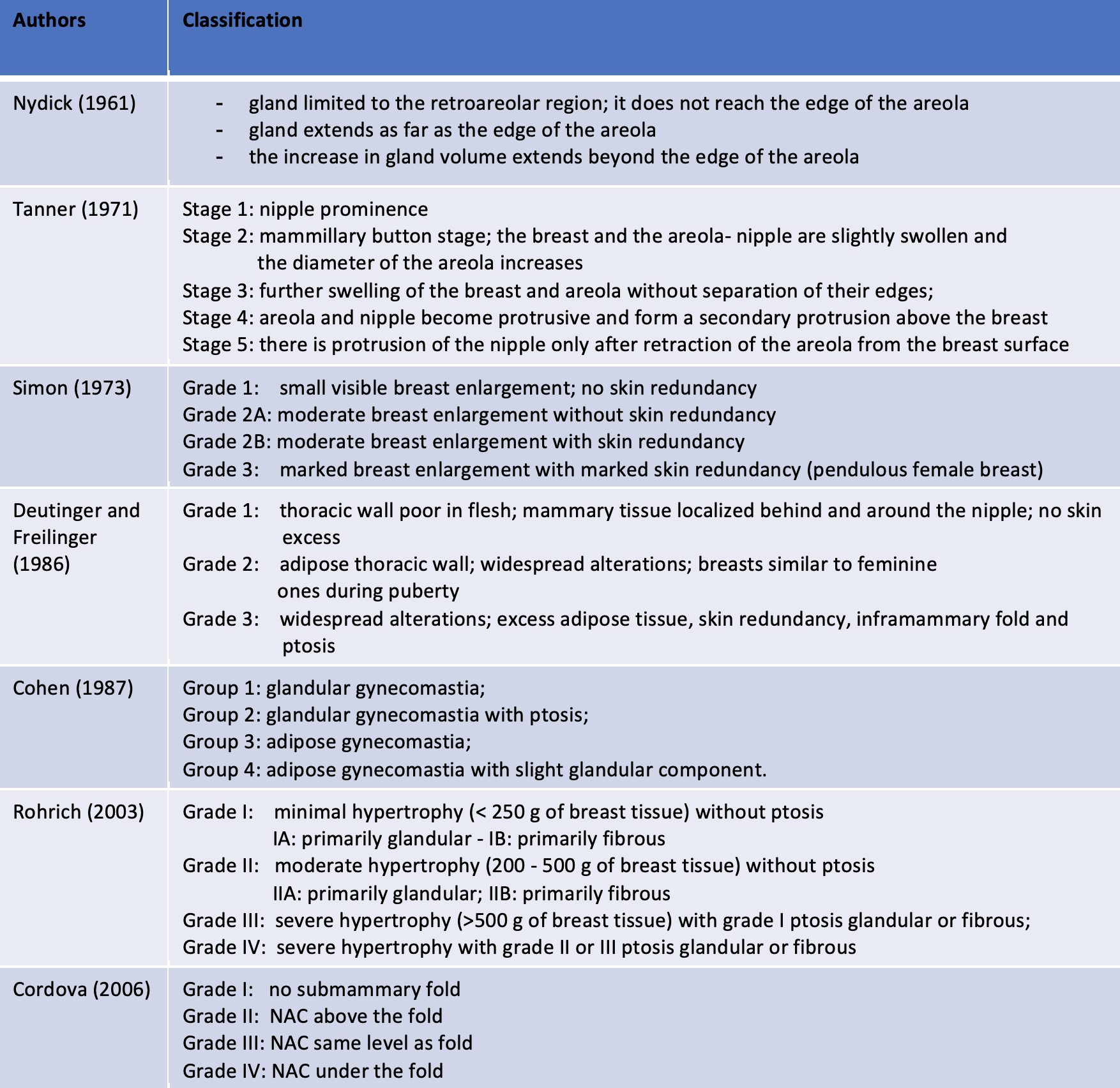
Classification History (modified from: Cordova et al.;J Plast Reconstr Aesthet Surg. 2008;61(1):41-9. doi: 10.1016)
Authors preferred Classification
The value of a classification is depending on its reproducible clinical implementation as well as on its medical consequences.
For a reproducible clinical assessment of gynecomastia particular limitations are the grading of skin laxity as well as the grading of fibrosis.
We stated that skin laxity after massive weight loss is highly reduced, and that one year after massive loss no further skin shrinking is expectable. Thus, we divided gynecomastia patients into two classes: with/without massive weight loss.
According to our preferred treatment algorithm subclasses with regards to fibrosis and skin excess were defined.
Although biopsy or ultrasound elastography could theoretically verify the grade of fibrosis subjective assessment remains as most practicable in clinical routine.
In patients without massive weight loss skin shrinking is expectable and thus should be awaited over 12 months.
If the MAC is stretchable 4 cm below the caudal border of the pectoralis major muscle the skin excess is sufficient to perform a horizontal mastectomy with fixation of the planned infrapectoral fold and transfer of the MAC 2 cm above.
In patients with massive weight loss and just moderate skin excess (not stretchable 4 cm below the pectoralis major muscle) a vertical skin excision is adequate.
For a reproducible clinical assessment of gynecomastia particular limitations are the grading of skin laxity as well as the grading of fibrosis.
We stated that skin laxity after massive weight loss is highly reduced, and that one year after massive loss no further skin shrinking is expectable. Thus, we divided gynecomastia patients into two classes: with/without massive weight loss.
According to our preferred treatment algorithm subclasses with regards to fibrosis and skin excess were defined.
Although biopsy or ultrasound elastography could theoretically verify the grade of fibrosis subjective assessment remains as most practicable in clinical routine.
In patients without massive weight loss skin shrinking is expectable and thus should be awaited over 12 months.
If the MAC is stretchable 4 cm below the caudal border of the pectoralis major muscle the skin excess is sufficient to perform a horizontal mastectomy with fixation of the planned infrapectoral fold and transfer of the MAC 2 cm above.
In patients with massive weight loss and just moderate skin excess (not stretchable 4 cm below the pectoralis major muscle) a vertical skin excision is adequate.
Authors preferred Classification
Histological Classification
Beside the division between the pseudo-gynecomastia (lipomatous gynecomastia) and true gynecomastia Bannayan et al. histologically classified the true gynecomastia [13]:
Histological type of gynecomastia
Composition
Florid gynecomastia
ductal hyperplasia and proliferation
Intermediate gynecomastia
ductal proliferation and stromal fibrosis
Fibrous gynecomastia
more stromal fibrosis
08
Conservative Treatment
Conservative Treatment
Due to the fact that gynecomastia is transient in 90% of adolescent patients it is recommended to wait until symptoms remain unchanged for 1-2 years before indicating any treatment [16, 18]. In cases where gynecomastia is induced by drugs or pathological reasons causative medication should be withdrawn and pathological findings should be addressed [16-19]. Thereby early acting within the early florid state of gynecomastia is crucial before glandular tissue will be replaced by irreversable fibrosis [16].
As conservative treatment pharmaceuticals can be highly effective before glandular fibrosis occurs.
Even not licensed for the treatment of gynecomastia Tamoxifen is the most popular medical treatment with response rates up to 95% [42-47].
The main indication for Tamoxifen is breast pain in gynecomastia smaller than 4 cm [19, 43].
Limiting side effects of Tamoxifen are[48]:
As conservative treatment pharmaceuticals can be highly effective before glandular fibrosis occurs.
Even not licensed for the treatment of gynecomastia Tamoxifen is the most popular medical treatment with response rates up to 95% [42-47].
The main indication for Tamoxifen is breast pain in gynecomastia smaller than 4 cm [19, 43].
Limiting side effects of Tamoxifen are[48]:
thromboembolic events (31%)
loss of libido (23%)
bone pain (15%)
neurocognitive deficits (15%)
leg cramps (8%)
ocular events (8%)
09
Operative Treatment
Operative Treatment
Liposuction
Conventional suction assisted liposuction (SAL) or water-jet assisted liposuction (WAL) are suitable to address the fatty tissue around the glandular mass:
Preoperative marking in standing position with respect to the extent of gynecomastia (head, body, tail), course of the pectoralis major muscle and axillary adhesion zones
General or local anesthesia
Supine position with arms abducted
Short lateral incision using an 11 blade within the infra-pectoral fold
In combination with a surgical excision two further periareolar incisions (3:00/09:00) can be useful
Liposuction using 4.2 and 3.5 mm cannulas (3.5mm for final conturing)
In case of extensiv liposuction placement of drains using the lateral incisions may be reasonable
If available, the treatment of the glandular mass by the use of ultrasound assisted liposuction (UAL) is reported to be highly effective [18, 48].
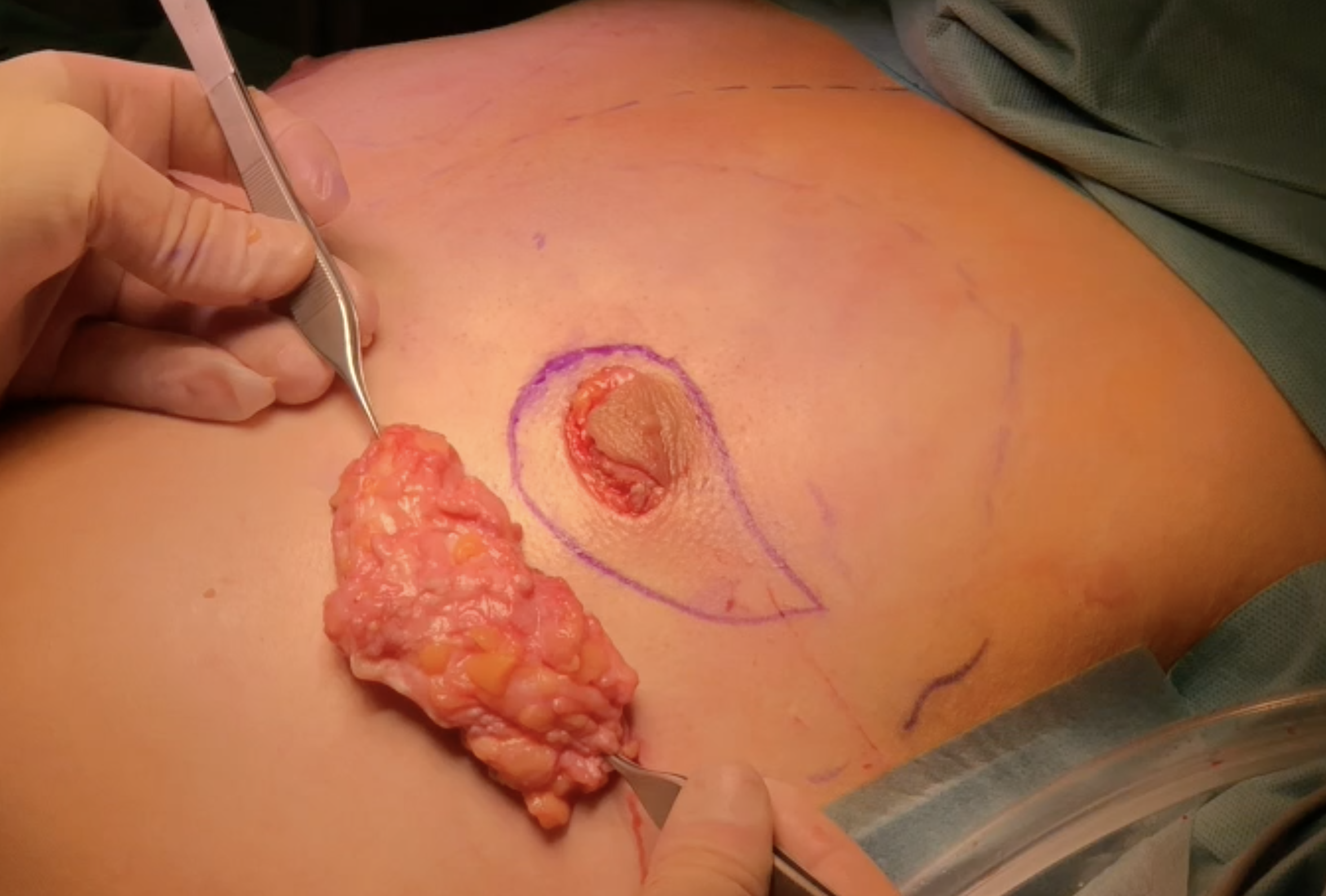
Subcutaneous mastectomy via periareolar incision
Periareolar and vertical skin excision (moderate skin excess after massive weight loss)
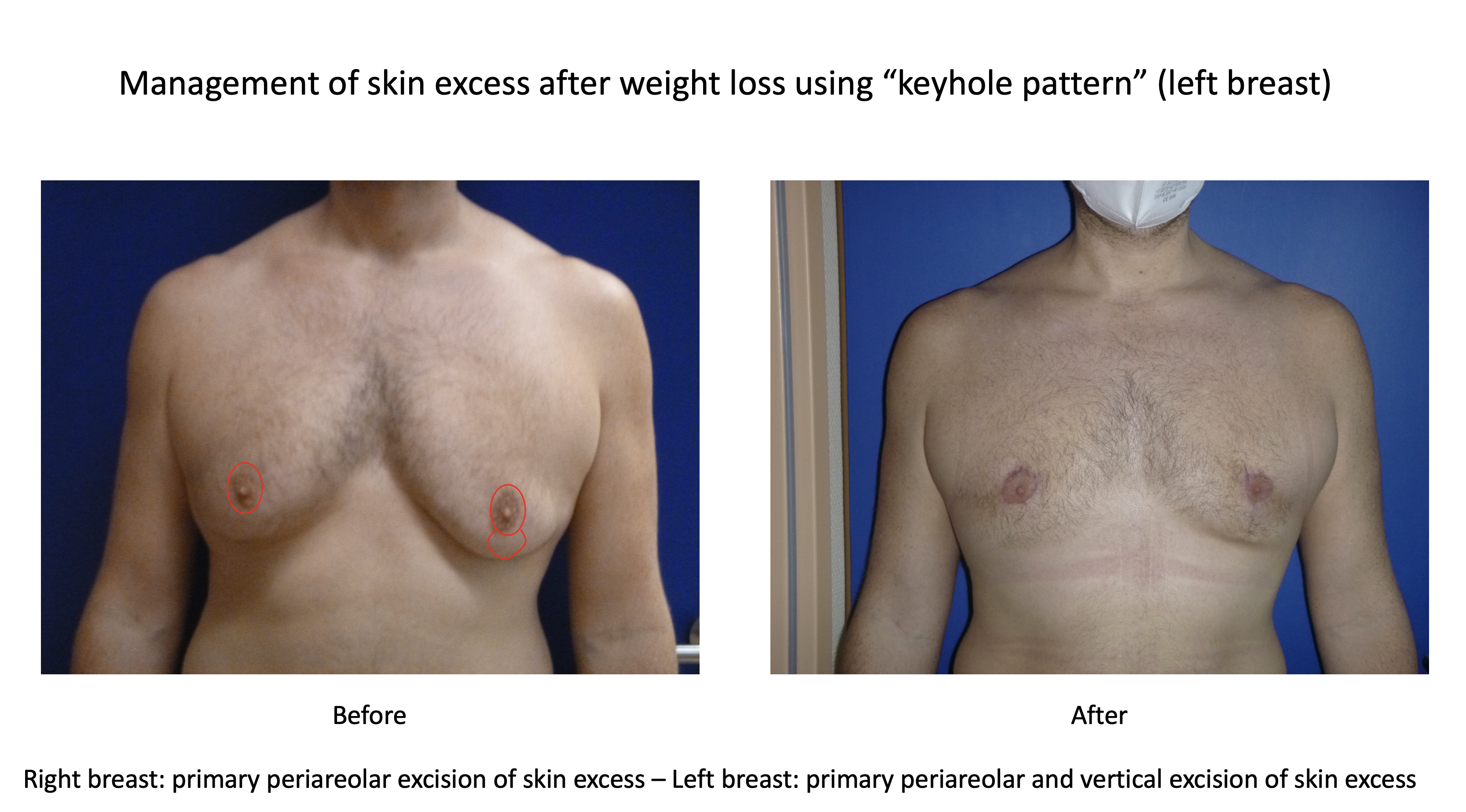
Management of skin excess using keyhole pattern
Horizontal mastectomy
10
Perioperative management
Perioperative management
Preoperative management
Discontinuation or conversion of anticoagulants - if possible
Nicotine abstinence 6 weeks before and after surgery
Customization of a compression vest or other compression garment
Postoperative Management
Inpatient observation over 1 night with regards to secondary bleeding
Immediate compression therapy after surgery (compressive dressing or garment) for 6 weeks
Drain removal when output <30cc / 24h - if placed.
Weekly follow-up care over 4 weeks with regards to seroma - if required puncture aspiration.
11
Common complications and their management
Common complications and their management
Hematoma
Cause: Bleeding, predominantly lateral out of branches of the lateral thoracic artery
Prevention: Raising blood pressure before diathermy (systole above 120 mmHg) and use of drains
Problem solution: Surgical revision, if possible aspiration puncture and conpression
Seroma
Cause: Collection of serous fluid due to inflammatory reaction after surgery
Prevention and solution: Compression garment for 6 weeks - weekly follow-up with regards to seroma, if required aspiration puncture.
Saucer deformity:
Cause: Glandular excision directly beneath areola, big glandular mass
Prevention: Leave a tissue layer of 1-2 cm beneath areola during subcutaneous mastectomy, alignment to surrounding tissue by liposuction
Problem solution: Alignment to surrounding tissue by liposuction, if required retroareolar lipofilling
Horizontal deformity of the NAC
Cause: Too much periareolar skin excision lateral and medial
Prevention: periareolar resection pattern should be vertical-oval
Problem solution: wait and see, if required corrective periareolar cephalad and caudal resection or lipofilling
Periareolar wound healing disturbance
Cause: Too much skin resection
Prevention: Secondary skin excision (12 months after primary surgery) respectively conservative skin excision
Problem solution: wait secondary healing, if required subsequent revision
Non-alignment of scar and infra-pectoral fold after horizontal mastectomy
Cause: Cephalad distortion of the scar due to missing infra-pectoral fixation
Prevention and solution: Infra-pectoral fixation using PDS sutures, if required infra-pectoral liposuction
12
References
1. Kinsella, C., Jr., et al., The psychological burden of idiopathic adolescent gynecomastia. Plast Reconstr Surg, 2012. 129(1): p. 1-7.2. Beer, G.M., et al., Configuration and localization of the nipple-areola complex in men. Plast Reconstr Surg, 2001. 108(7): p. 1947-52; discussion 1953.3. Agarwal, C.A., et al., Creation of an Aesthetic Male Nipple Areolar Complex in Female-to-Male Transgender Chest Reconstruction. Aesthetic Plast Surg, 2017. 41(6): p. 1305-1310.4. Blau, M., R. Hazani, and D. Hekmat, Anatomy of the Gynecomastia Tissue and Its Clinical Significance. Plast Reconstr Surg Glob Open, 2016. 4(8): p. e854.5. Shulman, O., et al., Appropriate location of the nipple-areola complex in males. Plast Reconstr Surg, 2001. 108(2): p. 348-51.6. Wolter, A., et al., Sexual reassignment surgery in female-to-male transsexuals: an algorithm for subcutaneous mastectomy. J Plast Reconstr Aesthet Surg, 2015. 68(2): p. 184-91.7. Lo Russo, G., S. Tanini, and M. Innocenti, Masculine Chest-Wall Contouring in FtM Transgender: a Personal Approach. Aesthetic Plast Surg, 2017. 41(2): p. 369-374.8. Maas, M., et al., The Ideal Male Nipple-Areola Complex: A Critical Review of the Literature and Discussion of Surgical Techniques for Female-to-Male Gender-Confirming Surgery. Ann Plast Surg, 2020. 84(3): p. 334-340.9. Kasai, S., et al., An anatomic study of nipple position and areola size in Asian men. Aesthet Surg J, 2015. 35(2): p. NP20-7.10. Vaucher, R., et al., [Anatomical study of men's nipple areola complex]. Ann Chir Plast Esthet, 2016. 61(3): p. 206-11.11. Sarhadi, N.S., et al., An anatomical study of the nerve supply of the breast, including the nipple and areola. Br J Plast Surg, 1996. 49(3): p. 156-64.12. Misery, L. and M. Talagas, Innervation of the Male Breast: Psychological and Physiological Consequences. J Mammary Gland Biol Neoplasia, 2017. 22(2): p. 109-115.13. Iuanow, E., M. Kettler, and P.J. Slanetz, Spectrum of disease in the male breast. AJR Am J Roentgenol, 2011. 196(3): p. W247-59.14. Bannayan, G.A. and S.I. Hajdu, Gynecomastia: clinicopathologic study of 351 cases. Am J Clin Pathol, 1972. 57(4): p. 431-7.15. Fricke, A., et al., Gynecomastia: histological appearance in different age groups. J Plast Surg Hand Surg, 2018. 52(3): p. 166-171.16. Cuhaci, N., et al., Gynecomastia: Clinical evaluation and management. Indian J Endocrinol Metab, 2014. 18(2): p. 150-8.17. Johnson, R.E. and M.H. Murad, Gynecomastia: pathophysiology, evaluation, and management. Mayo Clin Proc, 2009. 84(11): p. 1010-5.18. Sansone, A., et al., Gynecomastia and hormones. Endocrine, 2017. 55(1): p. 37-44.19. Rohrich, R.J., et al., Classification and management of gynecomastia: defining the role of ultrasound-assisted liposuction. Plast Reconstr Surg, 2003. 111(2): p. 909-23; discussion 924-5.20. Thiruchelvam, P., et al., Gynaecomastia. BMJ, 2016. 354: p. i4833.21. Akgul, S., N. Kanbur, and O. Derman, Pubertal gynecomastia: what about the remaining 10%? J Pediatr Endocrinol Metab, 2014. 27(9-10): p. 1027-8.22. Biro, F.M., et al., Hormonal studies and physical maturation in adolescent gynecomastia. J Pediatr, 1990. 116(3): p. 450-5.23. LaFranchi, S.H., et al., Pubertal gynecomastia and transient elevation of serum estradiol level. Am J Dis Child, 1975. 129(8): p. 927-31.24. Ma, N.S. and M.E. Geffner, Gynecomastia in prepubertal and pubertal men. Curr Opin Pediatr, 2008. 20(4): p. 465-70.25. Niewoehner, C.B. and F.Q. Nuttal, Gynecomastia in a hospitalized male population. Am J Med, 1984. 77(4): p. 633-8.26. Wu, F.C., et al., Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab, 2008. 93(7): p. 2737-45.27. Smyth, C.M. and W.J. Bremner, Klinefelter syndrome. Arch Intern Med, 1998. 158(12): p. 1309-14.28. Tan, Y.K., C.R. Birch, and D. Valerio, Bilateral gynaecomastia as the primary complaint in hyperthyroidism. J R Coll Surg Edinb, 2001. 46(3): p. 176-7.29. Iglesias, P., J.J. Carrero, and J.J. Diez, Gonadal dysfunction in men with chronic kidney disease: clinical features, prognostic implications and therapeutic options. J Nephrol, 2012. 25(1): p. 31-42.30. Hendry, W.S., et al., Ultrasonic detection of occult testicular neoplasms in patients with gynaecomastia. Br J Radiol, 1984. 57(679): p. 571-2.31. Hernes, E.H., K. Harstad, and Fossa, Changing incidence and delay of testicular cancer in southern Norway (1981-1992). Eur Urol, 1996. 30(3): p. 349-57.32. Daniels, I.R. and G.T. Layer, Testicular tumours presenting as gynaecomastia. Eur J Surg Oncol, 2003. 29(5): p. 437-9.33. Harris, M., et al., Testicular tumour presenting as gynaecomastia. BMJ, 2006. 332(7545): p. 837.34. Dundar, B., et al., Leptin levels in boys with pubertal gynecomastia. J Pediatr Endocrinol Metab, 2005. 18(10): p. 929-34.35. Bowman, J.D., H. Kim, and J.J. Bustamante, Drug-induced gynecomastia. Pharmacotherapy, 2012. 32(12): p. 1123-40.36. Dickson, G., Gynecomastia. Am Fam Physician, 2012. 85(7): p. 716-22.37. Karavolos, S., et al., Male central hypogonadism secondary to exogenous androgens: a review of the drugs and protocols highlighted by the online community of users for prevention and/or mitigation of adverse effects. Clin Endocrinol (Oxf), 2015. 82(5): p. 624-32.38. Nuttall, F.Q., Gynecomastia. Mayo Clin Proc, 2010. 85(10): p. 961-2.39. Abdelwahab Yousef, A.J., Male Breast Cancer: Epidemiology and Risk Factors. Semin Oncol, 2017. 44(4): p. 267-272.40. Thomas, D.B., Breast cancer in men. Epidemiol Rev, 1993. 15(1): p. 220-31.41. Schanz, S., et al., S1-Leitlinie: Gynakomastie im Erwachsenenalter. J Dtsch Dermatol Ges, 2017. 15(4): p. 465-472.42. Alagaratnam, T.T., Idiopathic gynecomastia treated with tamoxifen: a preliminary report. Clin Ther, 1987. 9(5): p. 483-7.43. Derman, O., et al., Long-term follow-up of tamoxifen treatment in adolescents with gynecomastia. J Pediatr Endocrinol Metab, 2008. 21(5): p. 449-54.44. Derman, O., N.O. Kanbur, and T.E. Tokur, The effect of tamoxifen on sex hormone binding globulin in adolescents with pubertal gynecomastia. J Pediatr Endocrinol Metab, 2004. 17(8): p. 1115-9.45. James, R., F. Ahmed, and G. Cunnick, The efficacy of tamoxifen in the treatment of primary gynecomastia: an observational study of tamoxifen versus observation alone. Breast J, 2012. 18(6): p. 620-1.46. Konig, R., et al., [Treatment of marked gynecomastia in puberty with tamoxifen]. Klin Padiatr, 1987. 199(6): p. 389-91.47. Lawrence, S.E., et al., Beneficial effects of raloxifene and tamoxifen in the treatment of pubertal gynecomastia. J Pediatr, 2004. 145(1): p. 71-6.48. Pemmaraju, N., et al., Retrospective review of male breast cancer patients: analysis of tamoxifen-related side-effects. Ann Oncol, 2012. 23(6): p. 1471-4.49. Zocchi, M.L., Ultrasonic assisted lipoplasty. Technical refinements and clinical evaluations. Clin Plast Surg, 1996. 23(4): p. 575-98.
Images
Authors preferred Classification
Authors preferred operative management
Classification History (modified from: Cordova et al.;J Plast Reconstr Aesthet Surg. 2008;61(1):41-9. doi: 10.1016)
Anatomy male breast
Composition of the female an male breast
Shear wave elastography of the male gland
Body, head, tail of male gland
Clinical algorithm (modified from: Rohrich, R.J., et al., Plast Reconstr Surg, 2003. 111(2): p. 909-23; discussion 924-5.)
Female breast - Regular male breast - Gynecomastia
Management of skin excess using keyhole pattern
Ideal position of the male nipple-areola complex.- Modified from Beer et. al., by Given et al. Plastische Chirurgie, 16. Jahrgang Heft 2/2016
Videos
Gynecomastia - Preoperative marking for subcutaneous mastectomy
Gynecomastia - Surgery video for subcutaneous mastectomy
Gynecomastia - Preoperative marking for horizontal mastectomy + free areola transplantation
Gynecomastia - Surgery video for horizontal mastectomy + free areola transplantation
References