["41532ae3d4f101474aa22e7a73b8f5a0c2aedae9","3fc45fcb0150fc5ffe38505e522ee37edd4702aa","42b4be201f4766cc48df2bfcdd9dcd3e1c4d6a61"]
Implant-based breast reconstruction
1199
1199
Chapter reads
0
0
Chapter likes
0/10
Evidence score
18
18
Images included
00
0
Videos included
01
Introduction [4] [22] [7] [19]
The process of reconstruction of the female breast following mastectomy is a relatively recent development in reconstructive surgery. Before the 1970s, techniques for reconstructing the breast after breast ablation were primarily used for skin defect coverage.
The introduction of silicone implants by Cronin in 1962 marked the first instance where the reconstruction of the breast mound became possible. In the 1980s, Radovan introduced the first fillable expander for the pre-expansion of the soft tissue.
Despite the advent of fully autologous options for breast reconstruction, such as the free transplanted or pedicled lower abdominal flap (Hartrampf 1982), silicone implants remain one of the most commonly employed techniques for breast reconstruction.
The introduction of silicone implants by Cronin in 1962 marked the first instance where the reconstruction of the breast mound became possible. In the 1980s, Radovan introduced the first fillable expander for the pre-expansion of the soft tissue.
Despite the advent of fully autologous options for breast reconstruction, such as the free transplanted or pedicled lower abdominal flap (Hartrampf 1982), silicone implants remain one of the most commonly employed techniques for breast reconstruction.
02
Indications and contraindications [3] [17]
Indications for implant-based breast reconstruction
• Mastectomy following mammary carcinoma (primary or secondary)
• Prophylactic mastectomy
• Benign diseases of the breast (e.g. fibroadenosis)
• Prophylactic mastectomy
• Benign diseases of the breast (e.g. fibroadenosis)
(Relative or absolute) contraindications for implant-based breast reconstruction
• Previous or planned radiotherapy of the breast
• Insufficient skin envelope
• Pre-existing severe scar tissue
• Pre-existing burn injury of the skin envelope
• Patient demanding autologous tissue
• Insufficient skin envelope
• Pre-existing severe scar tissue
• Pre-existing burn injury of the skin envelope
• Patient demanding autologous tissue
03
Preoperative examination [17] [14] [11] [3]
Measurements of the breast
• Sternal notch-to-nipple-distance (= SN)
• Underbust measurement
• Overbust measurement
• Distance between the nipple and the inframammary fold (= NF)
• Areola size
• Breast volume (e. g. by using the app “breast volume calculator)
• Degree of ptosis
• Breast width
• Distance between sternal notch and line between the lowest points of the underbust
For further measurements of the breast, please refer to our chapter breast reduction. (See section “metrics and volumetry of the breast”)
• Underbust measurement
• Overbust measurement
• Distance between the nipple and the inframammary fold (= NF)
• Areola size
• Breast volume (e. g. by using the app “breast volume calculator)
• Degree of ptosis
• Breast width
• Distance between sternal notch and line between the lowest points of the underbust
For further measurements of the breast, please refer to our chapter breast reduction. (See section “metrics and volumetry of the breast”)
Pictures
The photos should be taken from the front view with the arms hanging and raised as well as from both sides with the arms hanging. Photographs should also be taken from a 45 degree angle from both sides. Furthermore a 3D image with a body scan is recommended.
Timing of Implant-based reconstruction
Implant-based reconstruction of the breast can be performed either as an immediate reconstruction or as a secondary reconstruction at a later date.
Generally, there are only a few contraindications for immediate reconstruction, such as reduced overall health, severe concurrent diseases, or advanced age. Additionally, in some patients, there may be no desire for reconstruction. In all of these cases, offering a consultation is always recommended.
Many clinics do not provide immediate reconstruction. As a result, patients often present to a plastic surgery department at a later date with the request for secondary reconstruction.
When planning a subsequent breast reconstruction, we consider a waiting period of at least six months after the mastectomy. In the case of previous radiotherapy, we advocate a cautious postponement of one year, as a longer time interval is essential for a comprehensive assessment of the radiation-induced damage and radiodermatitis. Only after such a thorough assessment can a decision be made as to whether reconstruction with implants is possible or whether the patient's own tissue needs to be used.
Generally, there are only a few contraindications for immediate reconstruction, such as reduced overall health, severe concurrent diseases, or advanced age. Additionally, in some patients, there may be no desire for reconstruction. In all of these cases, offering a consultation is always recommended.
Many clinics do not provide immediate reconstruction. As a result, patients often present to a plastic surgery department at a later date with the request for secondary reconstruction.
When planning a subsequent breast reconstruction, we consider a waiting period of at least six months after the mastectomy. In the case of previous radiotherapy, we advocate a cautious postponement of one year, as a longer time interval is essential for a comprehensive assessment of the radiation-induced damage and radiodermatitis. Only after such a thorough assessment can a decision be made as to whether reconstruction with implants is possible or whether the patient's own tissue needs to be used.
Planning Mastectomy
In addition to the criteria mentioned above, the extent of the skin resection and the skin incision of the mastectomy also play an important role.
The planned incision depends, among other things, on the tumor size and location, the grading, tumor distance to the skin or nipple and the breast shape (ptosis, projection).
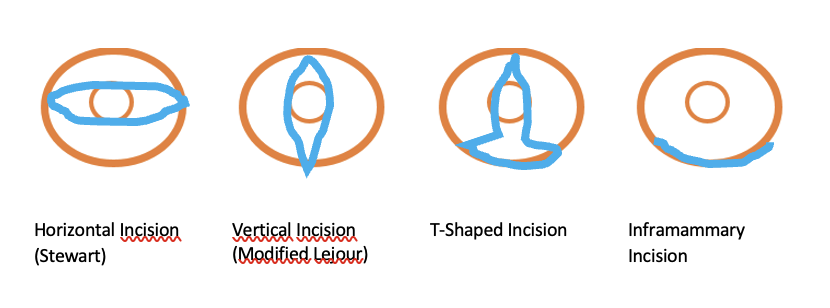
Variations of mastectomy / skin incisions:
Horizontal Incision (Stewart)
• Indication: standard approach for modified radical mastectomy including the resection of the nipple
• Advantages: Ensures high probability of complete tumor removal, simple surgical approach
• Disadvantages: Associated with partial loss of the skin mantle, diminished projection, occasional presence of excess lateral skin
Vertical Incision (Modified Lejour)
• Indication: Applied in the context of modified radical mastectomy,p rimarily employed for tumors situated cranially or caudally to the nipple and close to the skin
• Advantages: Preserves height integrity and promotes favorable projection outcomes
• Disadvantages: scar extending prominently towards the cranial aspect, potential for excess skin
T-Shaped Incision
• Indication: Primarily recommended for cases of macromastia and ptosis
• Advantages: Facilitates concurrent caudal dermis flap creation, ensuring an ample soft tissue mantle covering the caudal pole of the breast implant while simultaneously achieving tightening
• Disadvantage: time-consuming preparation, elevated risk of skin necrosis
Inframammary Incision
• Indication: Preferred for prophylactic mastectomy and cases involving small tumor sizes.
• Advantages: Affords complete preservation of the skin mantle.
• Disadvantage: Potential residual glandular remnants and the technically demanding preparation
The planned incision depends, among other things, on the tumor size and location, the grading, tumor distance to the skin or nipple and the breast shape (ptosis, projection).
Variations of mastectomy / skin incisions:
Horizontal Incision (Stewart)
• Indication: standard approach for modified radical mastectomy including the resection of the nipple
• Advantages: Ensures high probability of complete tumor removal, simple surgical approach
• Disadvantages: Associated with partial loss of the skin mantle, diminished projection, occasional presence of excess lateral skin
Vertical Incision (Modified Lejour)
• Indication: Applied in the context of modified radical mastectomy,p rimarily employed for tumors situated cranially or caudally to the nipple and close to the skin
• Advantages: Preserves height integrity and promotes favorable projection outcomes
• Disadvantages: scar extending prominently towards the cranial aspect, potential for excess skin
T-Shaped Incision
• Indication: Primarily recommended for cases of macromastia and ptosis
• Advantages: Facilitates concurrent caudal dermis flap creation, ensuring an ample soft tissue mantle covering the caudal pole of the breast implant while simultaneously achieving tightening
• Disadvantage: time-consuming preparation, elevated risk of skin necrosis
Inframammary Incision
• Indication: Preferred for prophylactic mastectomy and cases involving small tumor sizes.
• Advantages: Affords complete preservation of the skin mantle.
• Disadvantage: Potential residual glandular remnants and the technically demanding preparation
For further informations about mastectomy, please refer to our chapter indications for breast reconstruction. (See section “indication”)
04
Choice of technique [9] [3]
To decide whether an implant-based reconstruction or a reconstruction with autologous tissue should be performed, we refer to our general algorithm for breast reconstruction after mastectomy. (See chapter “indications for breast reconstruction”) The following algorithms refer to implant-based breast reconstruction in patients without radiotherapy.
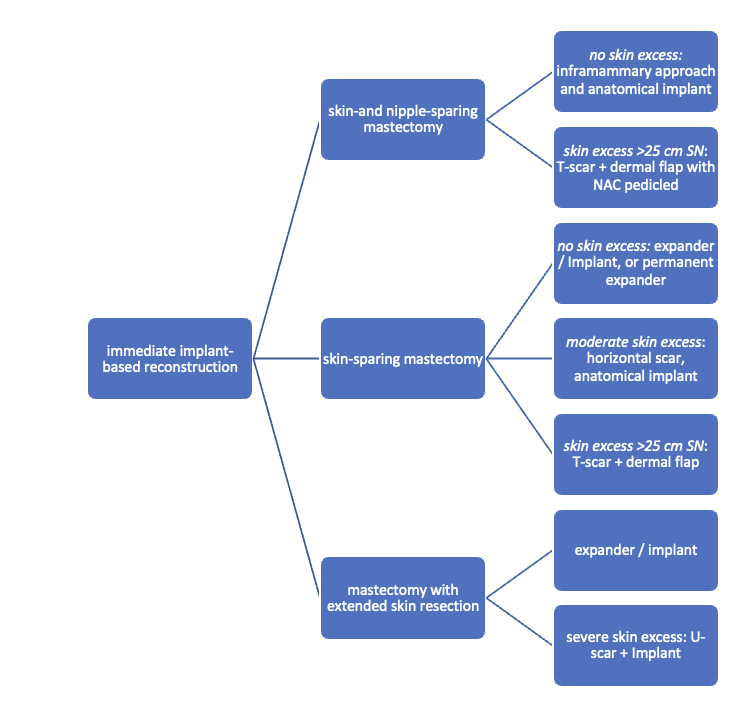
Immediate implant-based reconstruction
The determination of the extent of skin resection is a collaborative decision made in consultation with the oncological surgeon. Concurrently, the necessity for radiotherapy is also established in coordination with the oncological surgeon. This evaluation informs whether the existing skin mantle and soft tissue thickness are adequate for immediate reconstruction with a definitive implant or if pre-stretching using an expander is required.
The need for a skin-sparing or extended mastectomy is individually determined based on factors such as tumor type, tumor size and location, as well as tumor biology. Mastectomy procedures, particularly those aiming for complete nipple preservation, are often conducted through an incision in the lower breast crease. (A field hockey stick-shaped incision involving the edge of the nipple is also possible). When nipple resection is unavoidable, it is frequently satisfactory to include only the height of the areola in a transverse Stewart skin spindle, with a recommended height-to-width ratio not less than 1 : 3.
The feasibility of an immediate reconstruction utilizing a definitive implant can be approximated by initially summing the sternal notch-to-nipple distance (= SN) and the distance from the inframammary fold to the nipple. Subsequently, the height of the requisite resection spindle is subtracted from this total. Given an anticipated breast weight of approximately 500 g, the remaining distance should be at least 6 - 7 cm greater than the distance from the sternal notch to the line between the lowest points of the lower breast.
Algorithm for immediate implant-based reconstruction:
The need for a skin-sparing or extended mastectomy is individually determined based on factors such as tumor type, tumor size and location, as well as tumor biology. Mastectomy procedures, particularly those aiming for complete nipple preservation, are often conducted through an incision in the lower breast crease. (A field hockey stick-shaped incision involving the edge of the nipple is also possible). When nipple resection is unavoidable, it is frequently satisfactory to include only the height of the areola in a transverse Stewart skin spindle, with a recommended height-to-width ratio not less than 1 : 3.
The feasibility of an immediate reconstruction utilizing a definitive implant can be approximated by initially summing the sternal notch-to-nipple distance (= SN) and the distance from the inframammary fold to the nipple. Subsequently, the height of the requisite resection spindle is subtracted from this total. Given an anticipated breast weight of approximately 500 g, the remaining distance should be at least 6 - 7 cm greater than the distance from the sternal notch to the line between the lowest points of the lower breast.
Algorithm for immediate implant-based reconstruction:
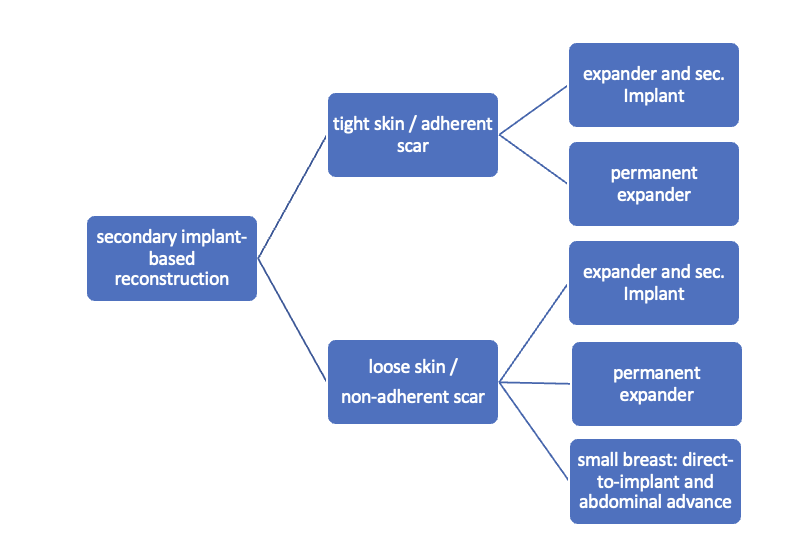
Secondary implant-based reconstruction
Secondary breast reconstruction employing an alloprosthesis typically involves the use of an expander, as there is rarely a sufficient skin mantle for a definitive implant. The selection of the implant site, whether subpectoral or epipectoral, is contingent upon the characteristics of the existing skin mantle. The application of a mesh for caudal coverage is generally unnecessary.
The underbust fold is usually no longer clearly defined and must be defined according to the opposite side, potentially through an internal pexy within the context of a localized abdominal wall lift.
Algorithm for secondary implant-based reconstruction:
The underbust fold is usually no longer clearly defined and must be defined according to the opposite side, potentially through an internal pexy within the context of a localized abdominal wall lift.
Algorithm for secondary implant-based reconstruction:
Caudal dermal flap
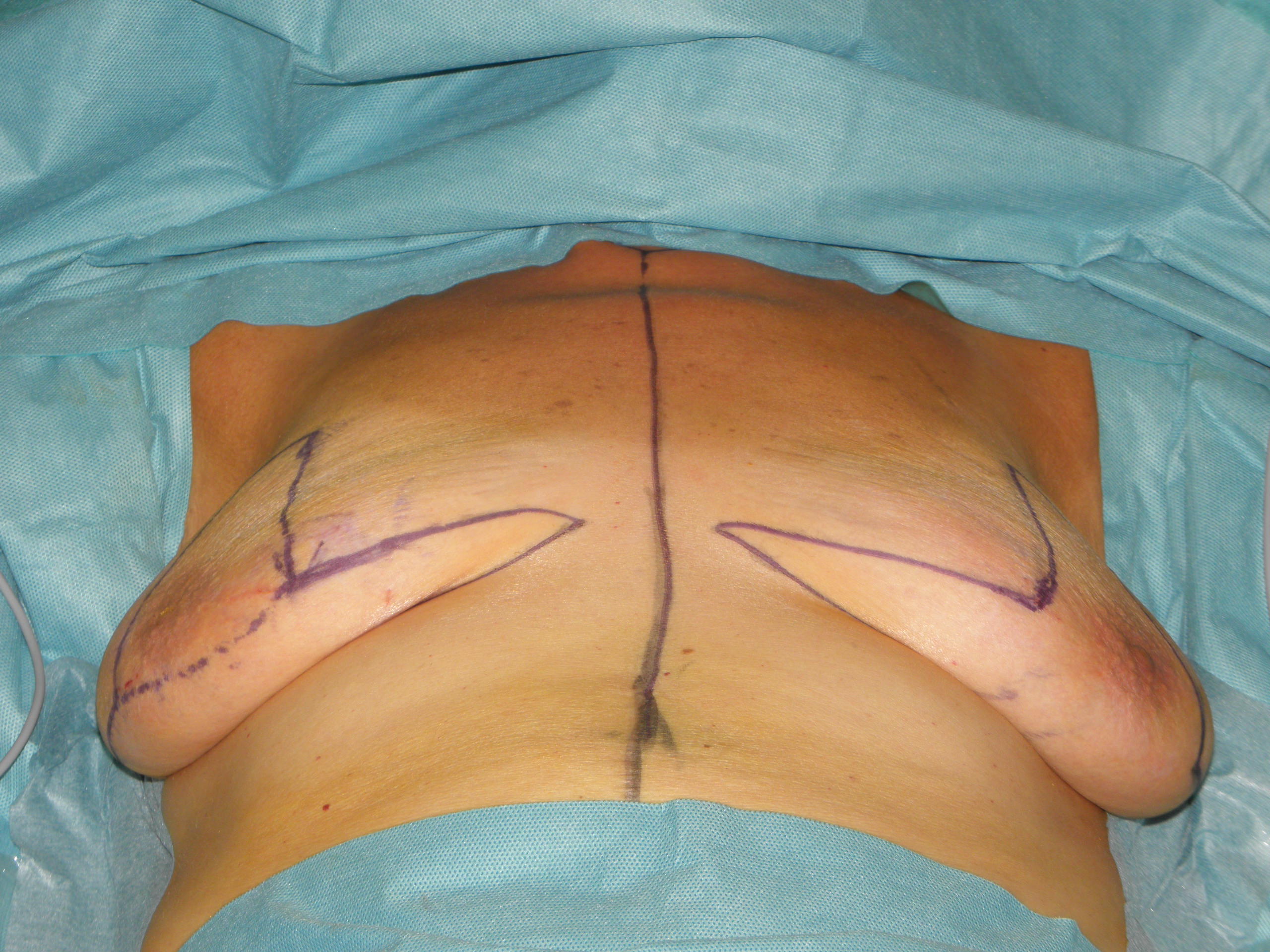
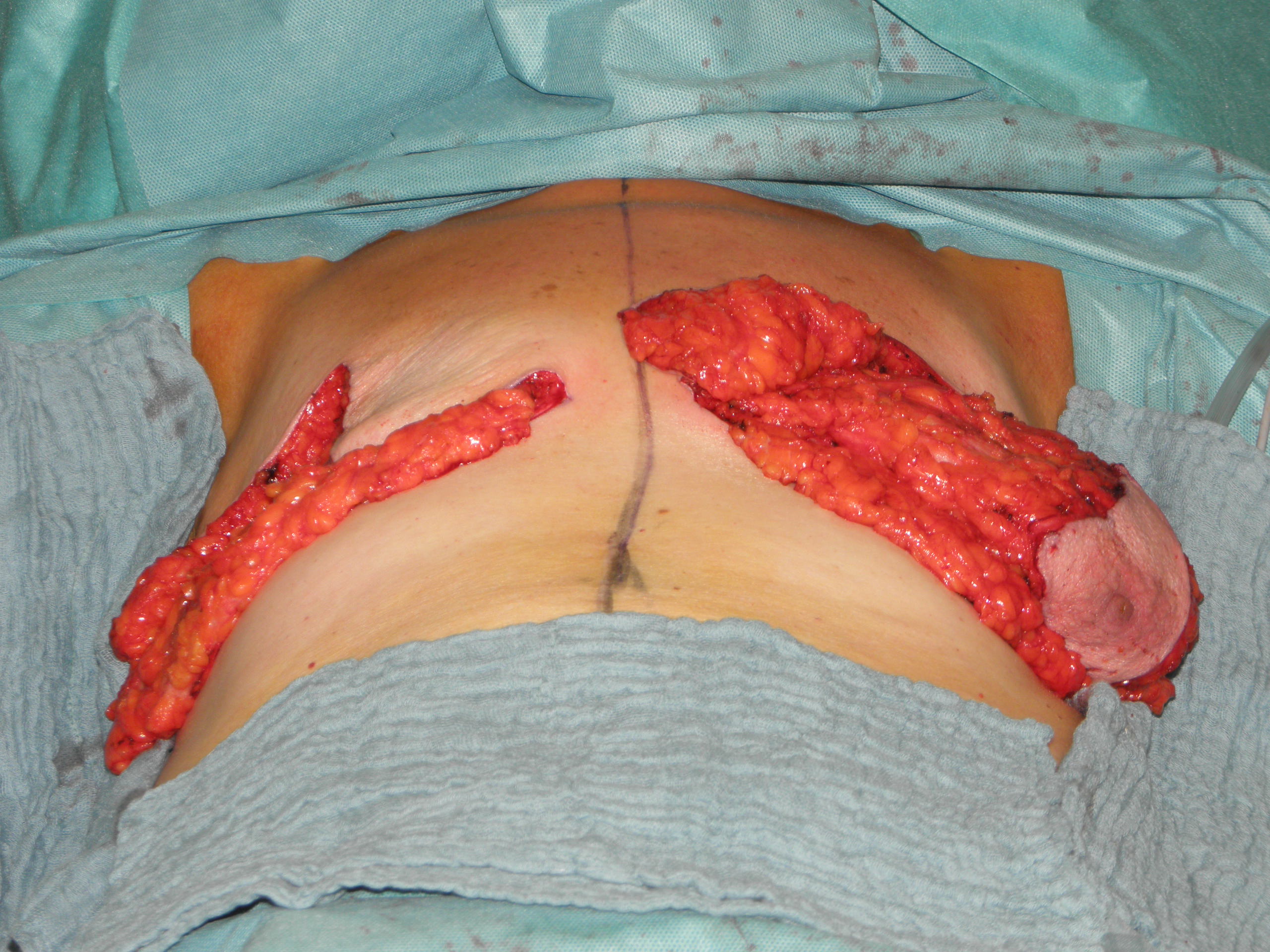
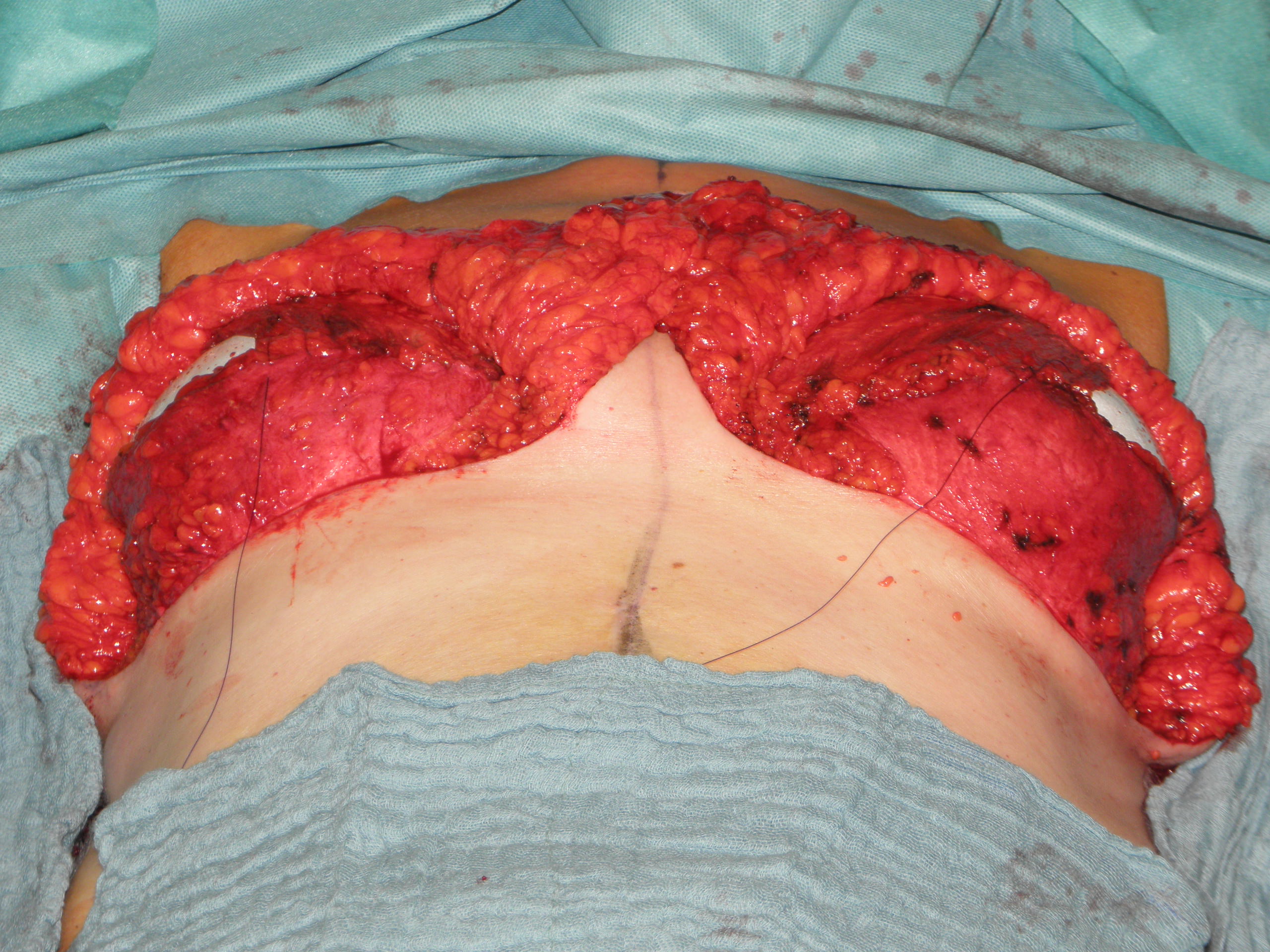
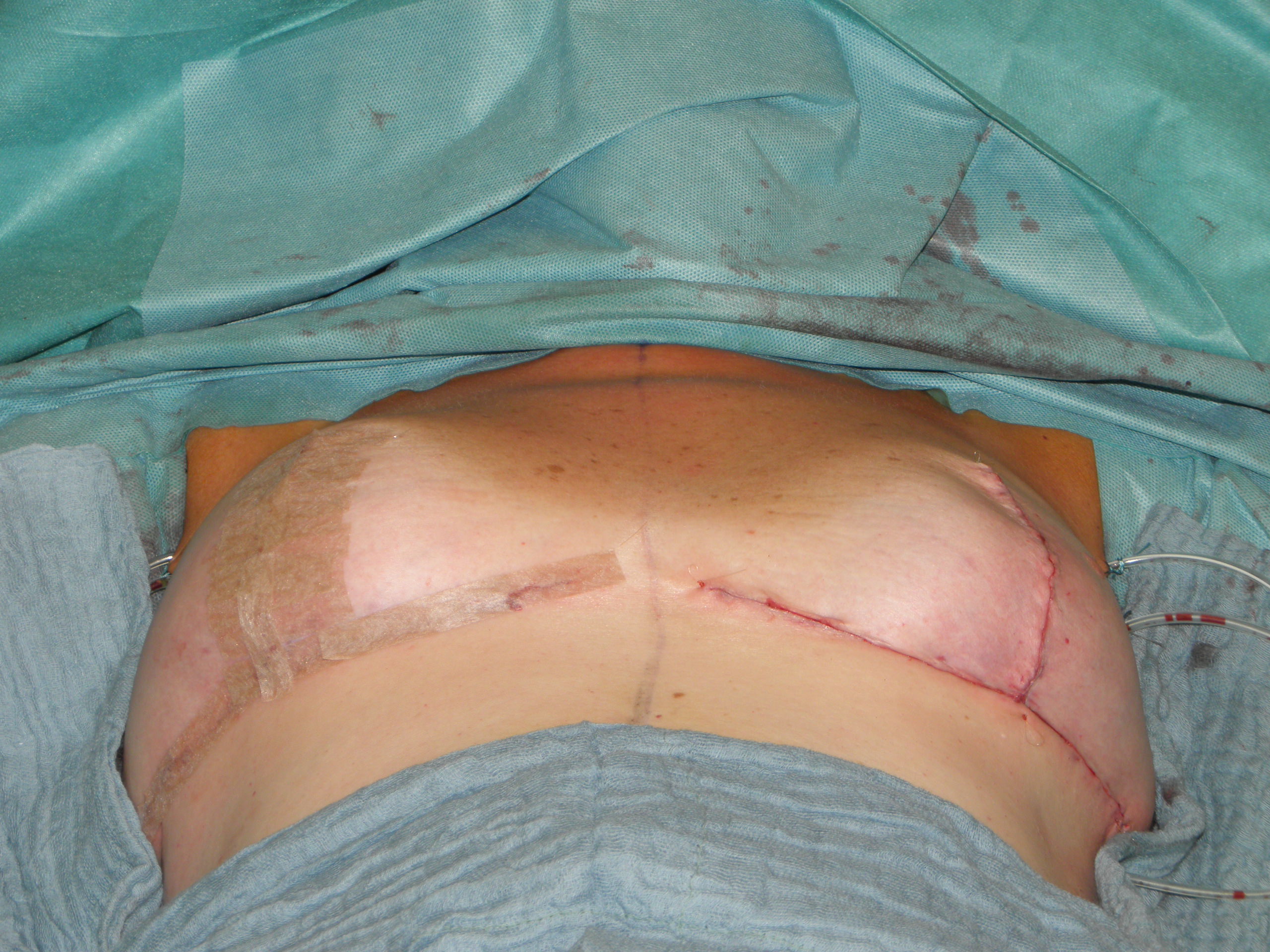
In case of skin excess/Ptosis or macromasty, the inferior quadrants of the potential skin envelope can be deepithelialized and used as an „internal bra“ to fixate the implant and secure the potential weak spots after T-scar incision.
Therefore the skin's caudal part has to be deepithelialized according to Wise pattern, creating a 5 mm thick adipodermal flap while preserving the subdermal vascular plexus. Following that subcutaneous mastectomy is performed, preserving the epipectoral fascia. Then the major pectoralis muscle is lifted, creating a subpectoral pocket for the insertion of a breast implant. The caudal dermis flap is sutured to the lower edge of the muscle, following the asymmetrical shape of the Wise pattern. To address the caudolateral deficit in coverage, the serratus fascia is mobilized laterally to the anterior axillary fold, completing the implant coverage by suturing it to the margin of the lateral pocket. The skin flaps are then placed over the vascularized pocket and closed in two layers after the insertion of a suction drainage. If the Nipple-Areola Complex (NAC) had not been removed, it has to be rotated cranially on its medial pedicle, positioned, and sutured to the point of the highest projection of the reconstructed breast.
Therefore the skin's caudal part has to be deepithelialized according to Wise pattern, creating a 5 mm thick adipodermal flap while preserving the subdermal vascular plexus. Following that subcutaneous mastectomy is performed, preserving the epipectoral fascia. Then the major pectoralis muscle is lifted, creating a subpectoral pocket for the insertion of a breast implant. The caudal dermis flap is sutured to the lower edge of the muscle, following the asymmetrical shape of the Wise pattern. To address the caudolateral deficit in coverage, the serratus fascia is mobilized laterally to the anterior axillary fold, completing the implant coverage by suturing it to the margin of the lateral pocket. The skin flaps are then placed over the vascularized pocket and closed in two layers after the insertion of a suction drainage. If the Nipple-Areola Complex (NAC) had not been removed, it has to be rotated cranially on its medial pedicle, positioned, and sutured to the point of the highest projection of the reconstructed breast.
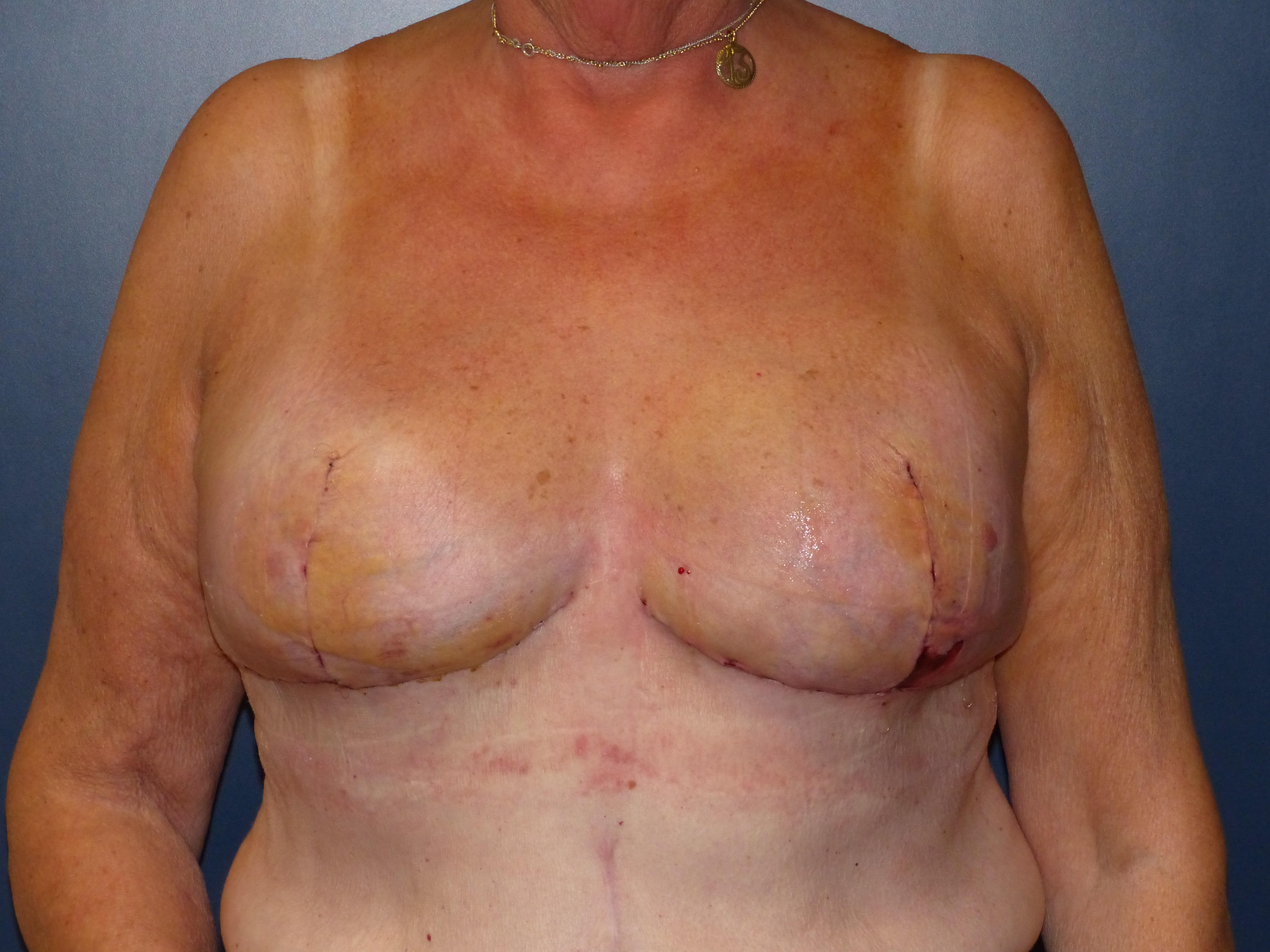
05
Choice of Implant [10] [19]
Relevant parameters
• Patient's constitution: pyknic / asthenic, soft tissue mantle, shape and size of the opposite breast
• Implant site : previous irradiation, previous surgery, pre-existing scars
• Desired size and shape of the breast (also consider volume of the resected tissue, pre-existing macromastia or ptosis)
• Implant site : previous irradiation, previous surgery, pre-existing scars
• Desired size and shape of the breast (also consider volume of the resected tissue, pre-existing macromastia or ptosis)
Expander
• Variants: temporary (two-stage) or permanent
• Form: round or anatomic shape
• Valve: integrated or separate
If a two-stage procedure with an expander and later replacement with a permanent breast implant is planned, the expander should be selected slightly larger than the volume of the resected breast tissue to receive an sufficient skin envelope. The selection of the expander depends on the breast width: for slim patients an expander with a round base is selected and for obese patients an expander with an oval base and a wider transverse diameter is selected. Notice that, the width of the expander in the epipectoral position should be approximately 1 cm narrower than the breast width (depending on the soft tissue mantle) and in the subpectoral position the width of the expander should be approximately 1,5 cm - 2 cm narrower than the breast width.In the case of pre-exsiting macromastia, the patient's wishes regarding the size should be taken into account and a reduction plastic surgery on the opposite side should be considered.
• Form: round or anatomic shape
• Valve: integrated or separate
If a two-stage procedure with an expander and later replacement with a permanent breast implant is planned, the expander should be selected slightly larger than the volume of the resected breast tissue to receive an sufficient skin envelope. The selection of the expander depends on the breast width: for slim patients an expander with a round base is selected and for obese patients an expander with an oval base and a wider transverse diameter is selected. Notice that, the width of the expander in the epipectoral position should be approximately 1 cm narrower than the breast width (depending on the soft tissue mantle) and in the subpectoral position the width of the expander should be approximately 1,5 cm - 2 cm narrower than the breast width.In the case of pre-exsiting macromastia, the patient's wishes regarding the size should be taken into account and a reduction plastic surgery on the opposite side should be considered.


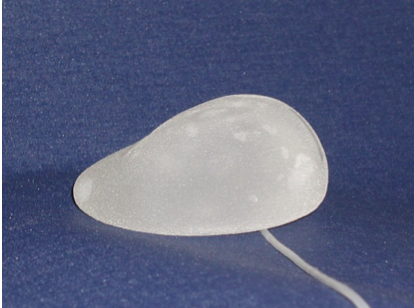
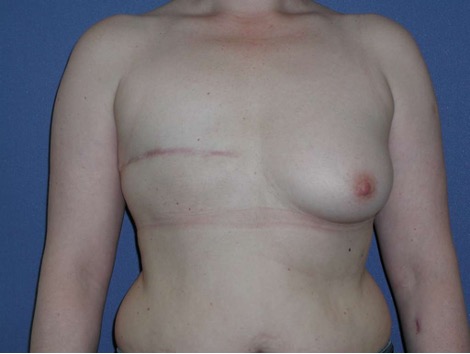
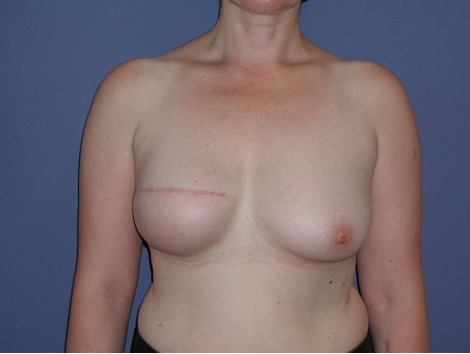
Permanent implant
• Form: Round or anatomic shape
• Surface: smooth, nanotextured, microtextured (50 ym)
• Consistency: different cohesive gels from soft to stabile; hybrid gel filling with two different gel types to support projection. Lightweight implants to reduce weight in case of higher volume.
In consultation with the patient, it is important establish whether the reconstruction goal involves replicating the original breast shape or pursuing a modified form.
Typically, for reconstructive interventions, an anatomical implant is preferred as the patient's own gland is missing to fortify projection. These implants should possess an adequately robust consistency. Occasionally, young and slender patients may express a preference for round implants. Such inclinations warrant consideration, particularly in cases with previous implant rotation.
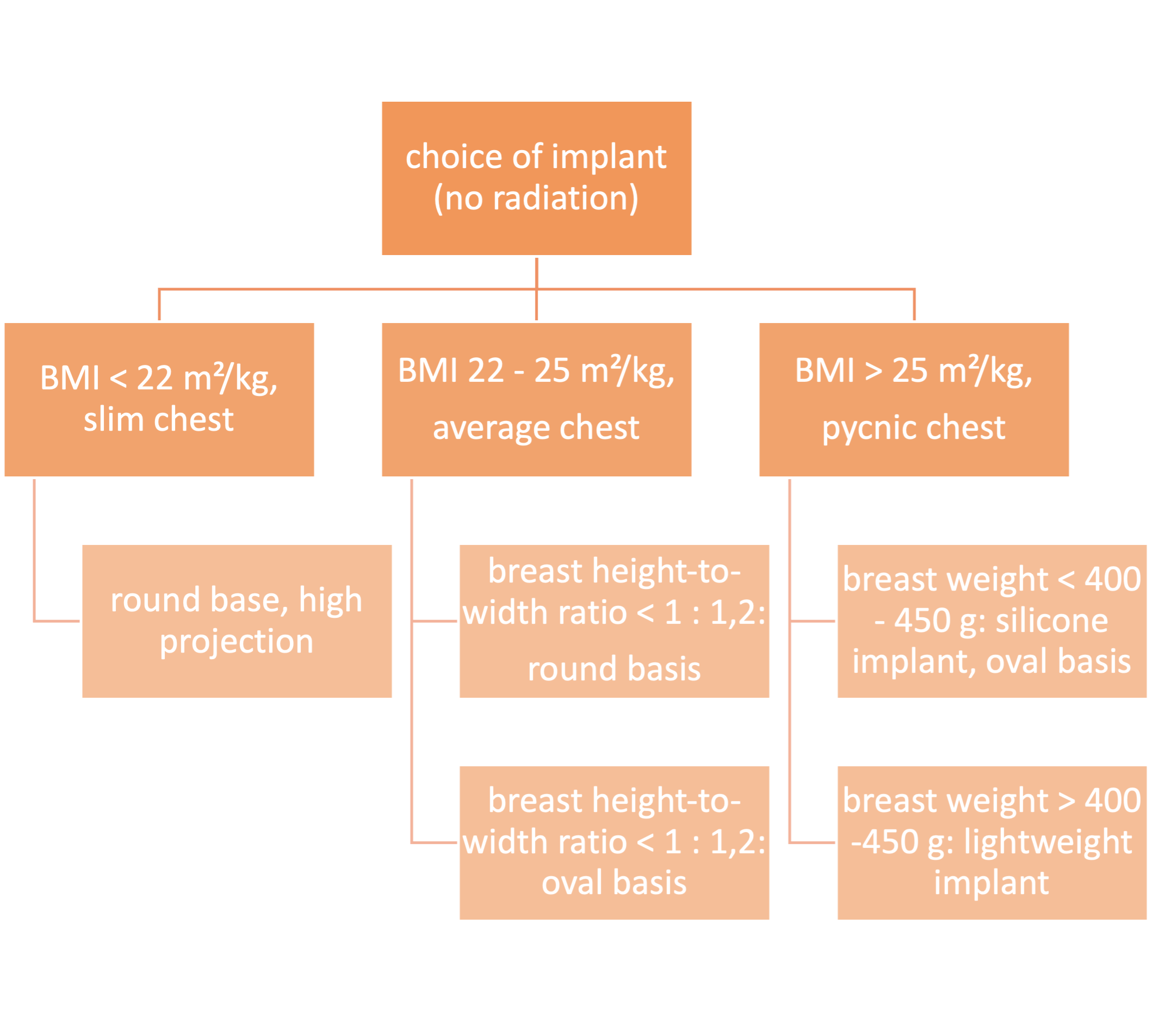
For slim patients with a BMI < 22 - 24 m²/kg and an original (or desired) breast shape featuring a height-to-width ratio of no more than 1 : 1,2, a round implant base is selected, otherwise, a transverse oval base is preferred. This is especially applicable in cases of ptosis mammae where no correction of the contralateral side is desired.
In the determination of projection, consideration must be given to the thickness of the subcutaneous mantle. (When in doubt, a high projection is recommended for obese patients.)
The utilization of lightweight implants (e.g., B-Lite, Polytech) may prove advantageous for volumes larger than 350 - 400 ml as their filling material is one third lighter than the conventional silicone gel used in conventional implants.
The use of a polyurethane foam-coated implant serves to mitigate the risk of recurrent capsular fibrosis, particularly in case of capsular contracture or when radiotherapy has been administered or is scheduled.
• Surface: smooth, nanotextured, microtextured (50 ym)
• Consistency: different cohesive gels from soft to stabile; hybrid gel filling with two different gel types to support projection. Lightweight implants to reduce weight in case of higher volume.
In consultation with the patient, it is important establish whether the reconstruction goal involves replicating the original breast shape or pursuing a modified form.
Typically, for reconstructive interventions, an anatomical implant is preferred as the patient's own gland is missing to fortify projection. These implants should possess an adequately robust consistency. Occasionally, young and slender patients may express a preference for round implants. Such inclinations warrant consideration, particularly in cases with previous implant rotation.
For slim patients with a BMI < 22 - 24 m²/kg and an original (or desired) breast shape featuring a height-to-width ratio of no more than 1 : 1,2, a round implant base is selected, otherwise, a transverse oval base is preferred. This is especially applicable in cases of ptosis mammae where no correction of the contralateral side is desired.
In the determination of projection, consideration must be given to the thickness of the subcutaneous mantle. (When in doubt, a high projection is recommended for obese patients.)
The utilization of lightweight implants (e.g., B-Lite, Polytech) may prove advantageous for volumes larger than 350 - 400 ml as their filling material is one third lighter than the conventional silicone gel used in conventional implants.
The use of a polyurethane foam-coated implant serves to mitigate the risk of recurrent capsular fibrosis, particularly in case of capsular contracture or when radiotherapy has been administered or is scheduled.



Algorithm for choice of implant:
06
Choice of implant site [3]
The optimal placement of implants for breast reconstruction, meaning whether they should be positioned under or above the muscle, is currently a topic of contentious debate.
Submuscular reconstruction offers advantages in terms of a more robust soft tissue cover, a smoother transition to the surrounding tissue, and enhanced protection against issues such as skin necrosis or wound healing disorders. Drawbacks include postoperative pain and potential movement of the implant during muscle activity.
The paramount consideration remains the secure removal of glandular tissue by the oncological surgeon. Compromises in the extent of gland resection to preserve a thicker soft tissue mantle for reconstruction are contraindicated.
The choice between submuscular and epimuscular reconstruction depends on the available soft tissue mantle and the size and shape of the resected of the mammary gland. In cases of uncertainty regarding the soft tissue mantle, indocyanine green-assisted laser angiography
Submuscular reconstruction offers advantages in terms of a more robust soft tissue cover, a smoother transition to the surrounding tissue, and enhanced protection against issues such as skin necrosis or wound healing disorders. Drawbacks include postoperative pain and potential movement of the implant during muscle activity.
The paramount consideration remains the secure removal of glandular tissue by the oncological surgeon. Compromises in the extent of gland resection to preserve a thicker soft tissue mantle for reconstruction are contraindicated.
The choice between submuscular and epimuscular reconstruction depends on the available soft tissue mantle and the size and shape of the resected of the mammary gland. In cases of uncertainty regarding the soft tissue mantle, indocyanine green-assisted laser angiography
07
Complications
General postoperative complications [6]
Common complications associated with implant-based breast reconstruction include
• Peri-implant fluid collection
• Hematoma
• Infection
• Intra- or extracapsular rupture
• Reactive lymphadenopathy
• Fat necrosis
• Peri-implant fluid collection
• Hematoma
• Infection
• Intra- or extracapsular rupture
• Reactive lymphadenopathy
• Fat necrosis
Capsular fibrosis [1] [2] [8] [13] [15] [16] [12] [23]
Inserting non-biological materials into the body always leads to the formation of a capsule. So, a notable drawback in the context of alloplastic breast reconstruction is the potential emergence of capsular contracture. This condition involves the hardening and thickening of the capsule formed around the implant. Clinically, capsular contracture eventually leads to pain and deformation of the implant.
The pathogenesis and etiology of this complication are not conclusively clarified, with current literature indicating it as a multifactorial process. Risk factors for capsular contracture include previous instances of capsular contracture, contamination with biofilm-producing bacteria, infections in the surgical area, and immune reactions to foreign material. Furthermore, numerous studies demonstrate that radiation therapy also increases the risk of capsular contracture. Borelli et al. and Moyer et al. have shown that an implant capsule after alloplastic breast reconstruction in irradiated tissue histologically differs from an implant capsule in non-irradiated tissue. Capsules in irradiated tissue contain an increased number of fibroblasts, an increased number of macrophages, and exhibit an increased diameter of the capsule wall. Therefore, planned radiation therapy should be considered when choosing the breast reconstruction procedure.
The current classification of capsular fibrosis relies on the clinical Baker score, primarily based on visible and palpable breast tissue findings. Exact tissue morphology assessment is challenging, prompting the use of ultrasound diagnostics, including elastography, for additional insights. Elastography assessments can provide localized information about tissue density but according to the literature they exhibit only a partial correlation with the findings based on the Baker test. Therefore further investigations are necessary.
Classification of capsular contracture after prosthetic breast reconstruction:
The pathogenesis and etiology of this complication are not conclusively clarified, with current literature indicating it as a multifactorial process. Risk factors for capsular contracture include previous instances of capsular contracture, contamination with biofilm-producing bacteria, infections in the surgical area, and immune reactions to foreign material. Furthermore, numerous studies demonstrate that radiation therapy also increases the risk of capsular contracture. Borelli et al. and Moyer et al. have shown that an implant capsule after alloplastic breast reconstruction in irradiated tissue histologically differs from an implant capsule in non-irradiated tissue. Capsules in irradiated tissue contain an increased number of fibroblasts, an increased number of macrophages, and exhibit an increased diameter of the capsule wall. Therefore, planned radiation therapy should be considered when choosing the breast reconstruction procedure.
The current classification of capsular fibrosis relies on the clinical Baker score, primarily based on visible and palpable breast tissue findings. Exact tissue morphology assessment is challenging, prompting the use of ultrasound diagnostics, including elastography, for additional insights. Elastography assessments can provide localized information about tissue density but according to the literature they exhibit only a partial correlation with the findings based on the Baker test. Therefore further investigations are necessary.
Classification of capsular contracture after prosthetic breast reconstruction:
Class IA
absolutely natural, cannot tell breast was reconstructed
Class IB
soft, but the implant is detectable by physical examination or inspection because of mastectomy
Class II
Mildly firm reconstructed breast with an implant that may be visible and detectable by physical examination
Class III
Moderately firm reconstructed breast. The implant is readily detectable, but the result may still be acceptable.
Class IV
Severe capsular contracture with an unacceptable aesthetic outcome and/or significant patient symptoms requiring surgical intervention.
Animation deformity [5] [24] [18]
Animation deformity is an aesthetically displeasing complication that can occur following sub-pectoral breast reconstruction. It is a condition characterized by the movement of breast implants during contraction of the pectoralis muscle, resulting in a motion deformity. Precisely, the contraction of the pectoralis muscle leads to an upward and outward (superolateral) movement of the breast implant.
While initially observed in patients undergoing breast augmentation, animation deformity appears to be more prevalent and potentially more severe in patients undergoing breast reconstruction. Spear et al. reported that 15% of breast augmentation patients exhibited moderate to severe deformity. In contrast, studies focusing on reconstructive patients have reported rates of up to 80% experiencing moderate to severe deformity.
Thus far, the primary factor definitively associated with the development of animation deformity is the placement of the implant in a sub-pectoral position. Patient-related factors, including age, body mass index (BMI), handedness, and athleticism, have not demonstrated a correlation with the occurrence or severity of animation deformity. Similarly, surgical factors like implant size, implant type, mastectomy specimen weight, and perioperative factors such as radiation, infection, and seroma, have not shown a correlation with the development or extent of animation deformity. Fracol et al. identified that the division of the pectoralis muscle coupled with the placement of acellular dermal matrix (ADM) is the sole factor that exhibits a correlation with the escalating severity of animation deformity. This observation aligns with findings in breast augmentation literature, where there is a frequent emphasis on preserving inferior pectoralis fibers to mitigate the occurrence of animation deformity.
Although animation deformity has been more extensively studied in the context of breast augmentation, there is no conclusive evidence demonstrating that it significantly impacts patient satisfaction with the procedure. This is likely due to the comparatively lower severity of animation deformity observed in this patient group. In contrast, in the breast reconstruction population, where deformity tends to be more severe and asymmetry is more probable, the impact on patient satisfaction is potentially greater, given the higher likelihood of unilateral procedures.
Treatment options range from converting to a pre-pectoral plane to employing muscle-splitting techniques, selective nerve ablation, and Botox injections. Further research into the causes, implications, and methods to enhance pre-pectoral reconstruction is crucial for improving patient outcomes associated with this phenomenon.
While initially observed in patients undergoing breast augmentation, animation deformity appears to be more prevalent and potentially more severe in patients undergoing breast reconstruction. Spear et al. reported that 15% of breast augmentation patients exhibited moderate to severe deformity. In contrast, studies focusing on reconstructive patients have reported rates of up to 80% experiencing moderate to severe deformity.
Thus far, the primary factor definitively associated with the development of animation deformity is the placement of the implant in a sub-pectoral position. Patient-related factors, including age, body mass index (BMI), handedness, and athleticism, have not demonstrated a correlation with the occurrence or severity of animation deformity. Similarly, surgical factors like implant size, implant type, mastectomy specimen weight, and perioperative factors such as radiation, infection, and seroma, have not shown a correlation with the development or extent of animation deformity. Fracol et al. identified that the division of the pectoralis muscle coupled with the placement of acellular dermal matrix (ADM) is the sole factor that exhibits a correlation with the escalating severity of animation deformity. This observation aligns with findings in breast augmentation literature, where there is a frequent emphasis on preserving inferior pectoralis fibers to mitigate the occurrence of animation deformity.
Although animation deformity has been more extensively studied in the context of breast augmentation, there is no conclusive evidence demonstrating that it significantly impacts patient satisfaction with the procedure. This is likely due to the comparatively lower severity of animation deformity observed in this patient group. In contrast, in the breast reconstruction population, where deformity tends to be more severe and asymmetry is more probable, the impact on patient satisfaction is potentially greater, given the higher likelihood of unilateral procedures.
Treatment options range from converting to a pre-pectoral plane to employing muscle-splitting techniques, selective nerve ablation, and Botox injections. Further research into the causes, implications, and methods to enhance pre-pectoral reconstruction is crucial for improving patient outcomes associated with this phenomenon.
Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) [25] [21]
Implant-related tumors have been described for some years. Assessing the precise global number of cases is challenging due to incomplete or redundant unstructured reports in various databases. Furthermore, there is often insufficient information regarding implant history, clinical progression, and histology.
Sporadic ALCL of the breast is an exceptionally rare disease, typically of B-cell origin, with an incidence of about 0.037 per 1 million women. T-cell lymphomas are even rarer. In comparison, implant-associated ALCL, though still infrequent, occurs more often than sporadic forms. The distinction between the two remains challenging, even with advanced analysis techniques. Estimates for BIA-ALCL incidence differ, with rates such as 1:355 patients and 1:53300, depending on implant type.
The precise origin of the tumor remains unclear, with multiple hypotheses suggesting a multifactorial etiology. Immunostimulation through a subclinical chronic inflammatory response seems to be a significant factor. Potential triggers include implant-related factors such as abrasion of silicone particles, or allergic hypersensitivity. Furthermore inflammatory responses to the implant sheath may play an important role. The implant's surface structure, especially textured surfaces, may contribute to its development, but this is not definitively established. There is no standardized classification for implant surfaces, and the relationship between surface characteristics and the incidence of BIA-ALCL is inconsistent. Procedure-related factors, like bacterial contamination with specific pathogens and biofilm formation, are also considered. An Australian working group found increased specific pathogens in the capsules of BIA-ALCL patients compared to benign capsule fibrosis, but these findings are yet to be confirmed by other groups. The clinical relevance remains unclear, and the influence of specific pathogens is considered unlikely. Implant surface colonization leads to biofilm formation, causing subclinical inflammation, which may contribute to malignant transformation through chronic stimulation of T-cells. It is suspected that a higher surface relief could contain a higher bacterial load, increasing the risk of T-cell stimulation. Genetic characteristics of the patient leading to an altered immune response may also be a contributing factor. However, there is currently insufficient scientific evidence supporting any of these hypotheses.
BIA-ALCL can manifest as a limited or infiltrative seroma. Typically, a large seroma with tumor cells emerges at least one year post-implantation, commonly after 7 to 10 years. Approximately 8–24% of patients may have an isolated or concomitant solid tumor, while systemic symptoms are rare. Ultrasound is the primary diagnostic method, with NMR and PET-CT for further assessment. Diagnosis involves analyzing the seroma, requiring sufficient cell material for flow cytometry. Key markers for confirmation include CD30 and ALK. Assessing capsular infiltration is crucial for prognosis and treatment planning, involving at least 12 biopsies after capsule resection.
Guidelines recommend complete surgical resection, including the implant capsule, as the primary treatment for BIA-ALCL. Adjuvant therapy may be considered in incomplete resection or systemic involvement cases, following systemic ALCL treatment. Avoiding sentinel lymph node removal is advised, with biopsy for abnormal nodes. For bilateral occurrences, removal of the contralateral implant is recommended. Options for reconstruction exist, but no standardized international recommendations for these procedures or follow-up care currently exist.
Sporadic ALCL of the breast is an exceptionally rare disease, typically of B-cell origin, with an incidence of about 0.037 per 1 million women. T-cell lymphomas are even rarer. In comparison, implant-associated ALCL, though still infrequent, occurs more often than sporadic forms. The distinction between the two remains challenging, even with advanced analysis techniques. Estimates for BIA-ALCL incidence differ, with rates such as 1:355 patients and 1:53300, depending on implant type.
The precise origin of the tumor remains unclear, with multiple hypotheses suggesting a multifactorial etiology. Immunostimulation through a subclinical chronic inflammatory response seems to be a significant factor. Potential triggers include implant-related factors such as abrasion of silicone particles, or allergic hypersensitivity. Furthermore inflammatory responses to the implant sheath may play an important role. The implant's surface structure, especially textured surfaces, may contribute to its development, but this is not definitively established. There is no standardized classification for implant surfaces, and the relationship between surface characteristics and the incidence of BIA-ALCL is inconsistent. Procedure-related factors, like bacterial contamination with specific pathogens and biofilm formation, are also considered. An Australian working group found increased specific pathogens in the capsules of BIA-ALCL patients compared to benign capsule fibrosis, but these findings are yet to be confirmed by other groups. The clinical relevance remains unclear, and the influence of specific pathogens is considered unlikely. Implant surface colonization leads to biofilm formation, causing subclinical inflammation, which may contribute to malignant transformation through chronic stimulation of T-cells. It is suspected that a higher surface relief could contain a higher bacterial load, increasing the risk of T-cell stimulation. Genetic characteristics of the patient leading to an altered immune response may also be a contributing factor. However, there is currently insufficient scientific evidence supporting any of these hypotheses.
BIA-ALCL can manifest as a limited or infiltrative seroma. Typically, a large seroma with tumor cells emerges at least one year post-implantation, commonly after 7 to 10 years. Approximately 8–24% of patients may have an isolated or concomitant solid tumor, while systemic symptoms are rare. Ultrasound is the primary diagnostic method, with NMR and PET-CT for further assessment. Diagnosis involves analyzing the seroma, requiring sufficient cell material for flow cytometry. Key markers for confirmation include CD30 and ALK. Assessing capsular infiltration is crucial for prognosis and treatment planning, involving at least 12 biopsies after capsule resection.
Guidelines recommend complete surgical resection, including the implant capsule, as the primary treatment for BIA-ALCL. Adjuvant therapy may be considered in incomplete resection or systemic involvement cases, following systemic ALCL treatment. Avoiding sentinel lymph node removal is advised, with biopsy for abnormal nodes. For bilateral occurrences, removal of the contralateral implant is recommended. Options for reconstruction exist, but no standardized international recommendations for these procedures or follow-up care currently exist.
Breast implant-associated squamous cell carcinoma (SCC) [25]
Breast implant-associated squamous cell carcinoma (SCC) is an extremely rare disease associated with both textured and smooth implants. Cases typically involve the first prosthesis being implanted more than 10 years prior. The origin of squamous epithelium is unclear, with possibilities including metaplasia due to chronic inflammatory stimulus. Similar to BIA-ALCL, implant-associated SCC is linked to a late seroma, but histopathologically, there is usually a circumscribed tumor in addition to the periprosthetic seroma. Antibodies against cytokeratin should be included in the work-up.
Due to the rarity of cases, there is no clear consensus on treatment, but it is recognized as a highly aggressive tumor with poor prognosis. Rapid and radical treatment, such as en-bloc or R0 resection, is recommended. Adjuvant radiotherapy or radiochemotherapy may be added. Rapid progression with metastasis has been reported in some cases despite R0 resection with adjuvant therapy, and follow-up information is limited in several instances.
Due to the rarity of cases, there is no clear consensus on treatment, but it is recognized as a highly aggressive tumor with poor prognosis. Rapid and radical treatment, such as en-bloc or R0 resection, is recommended. Adjuvant radiotherapy or radiochemotherapy may be added. Rapid progression with metastasis has been reported in some cases despite R0 resection with adjuvant therapy, and follow-up information is limited in several instances.
08
Outlook to the future: Concept for a National Implant Registry [20] [26]
The introduction of silicone implants has caused uncertainty among patients, the public, and users. Despite various incidents, there has been a lack of significant steps to improve patient safety.
In response, the German Society of Plastic, Aesthetic, and Reconstructive Surgeons (DGPRÄC), in collaboration with the Ministry of Health, has developed a concept, outlined in the coalition agreement, to address these concerns. This envisions the establishment of a unified, secure input portal for all relevant professional societies through the public entity. Three data qualities are collected: safety data (mandatory), practitioner data (voluntary), and research data (voluntary, unless they are safety-related). This dataset would enable the early detection of rare adverse events.
Currently DGPRÄC is already in close dialogue with an international consortium of Plastic Surgery professional societies (ICOBRA) regarding this matter. The DGPRÄC's concept has been positively received in discussions with various stakeholders (professional societies, implant manufacturers, health insurance companies, hospitals and medical associations, authorities). It is now up to the policymakers to seize the significant opportunity presented by this groundwork and establish the necessary structures to further elaborate and realize this concept in detail
In response, the German Society of Plastic, Aesthetic, and Reconstructive Surgeons (DGPRÄC), in collaboration with the Ministry of Health, has developed a concept, outlined in the coalition agreement, to address these concerns. This envisions the establishment of a unified, secure input portal for all relevant professional societies through the public entity. Three data qualities are collected: safety data (mandatory), practitioner data (voluntary), and research data (voluntary, unless they are safety-related). This dataset would enable the early detection of rare adverse events.
Currently DGPRÄC is already in close dialogue with an international consortium of Plastic Surgery professional societies (ICOBRA) regarding this matter. The DGPRÄC's concept has been positively received in discussions with various stakeholders (professional societies, implant manufacturers, health insurance companies, hospitals and medical associations, authorities). It is now up to the policymakers to seize the significant opportunity presented by this groundwork and establish the necessary structures to further elaborate and realize this concept in detail
09
Postoperative Treatment
Expander
Consistent with our internal protocols, we propose the following guidelines for follow-up care:
• Regular wound checks and dressing changes every 2-3 days
• Removal of the suture material 14 days postoperatively
• Keep the wound dry until the sutures are removed
• Consistent wearing of the compression bra for a period of 6 weeks, if necessary, combination of the compression bra with a pressure-increasing pad in case of heavy wound secretion
• Consistent relief (no lifting of loads >3 kg) for 6 weeks
Moreover, our in-house standard includes regular appointments in our outpatient-clinic after 2 weeks, 3 weeks, 4 weeks and 6 weeks postoperatively to fill the expander and monitor the healing process. (The expander is filled with 50 ml - 100 ml saline solution under sterile conditions every week starting from the second postoperative week.)
• Regular wound checks and dressing changes every 2-3 days
• Removal of the suture material 14 days postoperatively
• Keep the wound dry until the sutures are removed
• Consistent wearing of the compression bra for a period of 6 weeks, if necessary, combination of the compression bra with a pressure-increasing pad in case of heavy wound secretion
• Consistent relief (no lifting of loads >3 kg) for 6 weeks
Moreover, our in-house standard includes regular appointments in our outpatient-clinic after 2 weeks, 3 weeks, 4 weeks and 6 weeks postoperatively to fill the expander and monitor the healing process. (The expander is filled with 50 ml - 100 ml saline solution under sterile conditions every week starting from the second postoperative week.)
Permanent implant
Consistent with our internal protocols, we propose the following guidelines for follow-up care:
• Regular wound checks and dressing changes every 2-3 days
• Removal of the suture material 12-14 days postoperatively
• Keep the wound dry until the sutures are removed
• Consistent wearing of the compression bra (with Stuttgart belt if necessary) for a period of 6 weeks
• Consistent weight relief (no lifting of loads >3 kg) for 6 weeks
• Avoid lifting the arms above shoulder height for 2 weeks
Moreover, our in-house standard includes regular appointments in our outpatient-clinic after 2 weeks and 6 weeks postoperatively to monitor the healing process.
• Regular wound checks and dressing changes every 2-3 days
• Removal of the suture material 12-14 days postoperatively
• Keep the wound dry until the sutures are removed
• Consistent wearing of the compression bra (with Stuttgart belt if necessary) for a period of 6 weeks
• Consistent weight relief (no lifting of loads >3 kg) for 6 weeks
• Avoid lifting the arms above shoulder height for 2 weeks
Moreover, our in-house standard includes regular appointments in our outpatient-clinic after 2 weeks and 6 weeks postoperatively to monitor the healing process.
10
References
1. Bayston R. (2022) Capsule formation around breast implants. JPRAS Open. 31: S. 123-128.
2. Borrelli M.R., Irizzary D., Patel R.A., Nguyen D., Momeni A., Longaker M.T., und Wan D.C. (2020) Pro-Fibrotic CD26-Positive Fibroblasts Are Present in Greater Abundance in Breast Capsule Tissue of Irradiated Breasts. Aesthet Surg J. 40: S. 369-379.
3. Colwell A.S. und Taylor E.M. (2020) Recent Advances in Implant-Based Breast Reconstruction. Plast Reconstr Surg. 145: S. 421e-432e.
4. Cronin T. und Gerow F. (1963) Transactions of the third international congress of plastic surgery.
5. Fracol M., Feld L.N., Chiu W.K., und Kim J.Y.S. (2019) An overview of animation deformity in prosthetic breast reconstruction. Gland Surg. 8: S. 95-101.
6. Georgieva M., Kammerer S., Prantl L., Jung F., Stroszczynski C., und Jung E.M. (2020) Imaging of breast implant and implant-associated complications: Capsular contracture and intra- or extracapsular rupture. Clin Hemorheol Microcirc. 76: S. 221-231.
7. Hartrampf C.R., Scheflan M., und Black P.W. (1982) Breast reconstruction with a transverse abdominal island flap. Plast Reconstr Surg. 69: S. 216-25.
8. Headon H., Kasem A., und Mokbel K. (2015) Capsular Contracture after Breast Augmentation: An Update for Clinical Practice. Arch Plast Surg. 42: S. 532-43.
9. Heine N., Hoesl V., Seitz S., Prantl L., und Brebant V. (2022) Implant-based immediate reconstruction in prophylactic mastectomy: is the caudal dermis flap a reliable alternative to synthetic mesh or acellular dermal matrix? Arch Gynecol Obstet. 305: S. 937-943.
10. Heine N., Brebant V., Seitz S., Eigenberger A., Prantl L., und Tessmann V. (2023) Lightweight implants in breast reconstruction. Clin Hemorheol Microcirc. 84: S. 103-109.
11. Huang N.S., Quan C.L., Mo M., Chen J.J., Yang B.L., Huang X.Y., und Wu J. (2017) A prospective study of breast anthropomorphic measurements, volume and ptosis in 605 Asian patients with breast cancer or benign breast disease. PLoS One. 12: S. e0172122.
12. Jung E., Hosl V., von Fraunberg S., Jung F., und Prantl L. (2021) Ultrasound elastography for the detection of capsular fibrosis in breast implants: First results. Clin Hemorheol Microcirc. 77: S. 247-257.
13. Larsen A., Rasmussen L.E., Rasmussen L.F., Weltz T.K., Hemmingsen M.N., Poulsen S.S., Jacobsen J.C.B., Vester-Glowinski P., und Herly M. (2021) Histological Analyses of Capsular Contracture and Associated Risk Factors: A Systematic Review. Aesthetic Plast Surg. 45: S. 2714-2728.
14. Longo B., Farcomeni A., Ferri G., Campanale A., Sorotos M., und Santanelli F. (2013) The BREAST-V: a unifying predictive formula for volume assessment in small, medium, and large breasts. Plast Reconstr Surg. 132: S. 1e-7e.
15. Luvsannyam E., Patel D., Hassan Z., Nukala S., Somagutta M.R., und Hamid P. (2020) Overview of Risk Factors and Prevention of Capsular Contracture Following Implant-Based Breast Reconstruction and Cosmetic Surgery: A Systematic Review. Cureus. 12: S. e10341.
16. Moyer H.R., Pinell-White X., und Losken A. (2014) The effect of radiation on acellular dermal matrix and capsule formation in breast reconstruction: clinical outcomes and histologic analysis. Plast Reconstr Surg. 133: S. 214-221.
17. Nahabedian M.Y. (2016) Implant-based breast reconstruction following conservative mastectomy: one-stage vs. two-stage approach. Gland Surg. 5: S. 47-54.
18. Nigro L.C. und Blanchet N.P. (2017) Animation Deformity in Postmastectomy Implant-Based Reconstruction. Plast Reconstr Surg Glob Open. 5: S. e1407.
19. Perry D. und Frame J.D. (2020) The history and development of breast implants. Ann R Coll Surg Engl. 102: S. 478-482.
20. Prantl L., von Fritschen U., Liebau J., von Hassel J., Baur E.M., Vogt P.M., Giunta R.E., und Horch R.E. (2016) [Concept for a National Implant Registry to Improve Patient Safety]. Handchir Mikrochir Plast Chir. 48: S. 320-329.
21. Prantl L., Gerken M., Zeman F., Leitzmann M., Koller M., Klinkhammer-Schalke M., Evert M., Kuehlmann B., und Biermann N. (2020) Incidence of Anaplastic Large Cell Lymphoma and Breast-Implant-Associated Lymphoma-An Analysis of a Certified Tumor Registry over 17 Years. J Clin Med. 9.
22. Radovan C. (1982) Breast reconstruction after mastectomy using the temporary expander. Plast Reconstr Surg. 69: S. 195-208.
23. Spear S.L. und Baker J.L., Jr. (1995) Classification of capsular contracture after prosthetic breast reconstruction. Plast Reconstr Surg. 96: S. 1119-23; discussion 1124.
24. Spear S.L., Schwartz J., Dayan J.H., und Clemens M.W. (2009) Outcome assessment of breast distortion following submuscular breast augmentation. Aesthetic Plast Surg. 33: S. 44-8.
25. von Fritschen U., Kremer T., Prantl L., und Fricke A. (2023) Breast Implant-Associated Tumors. Geburtshilfe Frauenheilkd. 83: S. 686-693.
26. von Fritschen U., Rakhorst H.A., Stark B., Ahern S., Prantl L., und Fricke A. (2023) The Mandatory German Breast Implant Registry Law: A Model for Sustainable Implant Registries. Aesthet Surg J. 43: S. NP858-NP865.
2. Borrelli M.R., Irizzary D., Patel R.A., Nguyen D., Momeni A., Longaker M.T., und Wan D.C. (2020) Pro-Fibrotic CD26-Positive Fibroblasts Are Present in Greater Abundance in Breast Capsule Tissue of Irradiated Breasts. Aesthet Surg J. 40: S. 369-379.
3. Colwell A.S. und Taylor E.M. (2020) Recent Advances in Implant-Based Breast Reconstruction. Plast Reconstr Surg. 145: S. 421e-432e.
4. Cronin T. und Gerow F. (1963) Transactions of the third international congress of plastic surgery.
5. Fracol M., Feld L.N., Chiu W.K., und Kim J.Y.S. (2019) An overview of animation deformity in prosthetic breast reconstruction. Gland Surg. 8: S. 95-101.
6. Georgieva M., Kammerer S., Prantl L., Jung F., Stroszczynski C., und Jung E.M. (2020) Imaging of breast implant and implant-associated complications: Capsular contracture and intra- or extracapsular rupture. Clin Hemorheol Microcirc. 76: S. 221-231.
7. Hartrampf C.R., Scheflan M., und Black P.W. (1982) Breast reconstruction with a transverse abdominal island flap. Plast Reconstr Surg. 69: S. 216-25.
8. Headon H., Kasem A., und Mokbel K. (2015) Capsular Contracture after Breast Augmentation: An Update for Clinical Practice. Arch Plast Surg. 42: S. 532-43.
9. Heine N., Hoesl V., Seitz S., Prantl L., und Brebant V. (2022) Implant-based immediate reconstruction in prophylactic mastectomy: is the caudal dermis flap a reliable alternative to synthetic mesh or acellular dermal matrix? Arch Gynecol Obstet. 305: S. 937-943.
10. Heine N., Brebant V., Seitz S., Eigenberger A., Prantl L., und Tessmann V. (2023) Lightweight implants in breast reconstruction. Clin Hemorheol Microcirc. 84: S. 103-109.
11. Huang N.S., Quan C.L., Mo M., Chen J.J., Yang B.L., Huang X.Y., und Wu J. (2017) A prospective study of breast anthropomorphic measurements, volume and ptosis in 605 Asian patients with breast cancer or benign breast disease. PLoS One. 12: S. e0172122.
12. Jung E., Hosl V., von Fraunberg S., Jung F., und Prantl L. (2021) Ultrasound elastography for the detection of capsular fibrosis in breast implants: First results. Clin Hemorheol Microcirc. 77: S. 247-257.
13. Larsen A., Rasmussen L.E., Rasmussen L.F., Weltz T.K., Hemmingsen M.N., Poulsen S.S., Jacobsen J.C.B., Vester-Glowinski P., und Herly M. (2021) Histological Analyses of Capsular Contracture and Associated Risk Factors: A Systematic Review. Aesthetic Plast Surg. 45: S. 2714-2728.
14. Longo B., Farcomeni A., Ferri G., Campanale A., Sorotos M., und Santanelli F. (2013) The BREAST-V: a unifying predictive formula for volume assessment in small, medium, and large breasts. Plast Reconstr Surg. 132: S. 1e-7e.
15. Luvsannyam E., Patel D., Hassan Z., Nukala S., Somagutta M.R., und Hamid P. (2020) Overview of Risk Factors and Prevention of Capsular Contracture Following Implant-Based Breast Reconstruction and Cosmetic Surgery: A Systematic Review. Cureus. 12: S. e10341.
16. Moyer H.R., Pinell-White X., und Losken A. (2014) The effect of radiation on acellular dermal matrix and capsule formation in breast reconstruction: clinical outcomes and histologic analysis. Plast Reconstr Surg. 133: S. 214-221.
17. Nahabedian M.Y. (2016) Implant-based breast reconstruction following conservative mastectomy: one-stage vs. two-stage approach. Gland Surg. 5: S. 47-54.
18. Nigro L.C. und Blanchet N.P. (2017) Animation Deformity in Postmastectomy Implant-Based Reconstruction. Plast Reconstr Surg Glob Open. 5: S. e1407.
19. Perry D. und Frame J.D. (2020) The history and development of breast implants. Ann R Coll Surg Engl. 102: S. 478-482.
20. Prantl L., von Fritschen U., Liebau J., von Hassel J., Baur E.M., Vogt P.M., Giunta R.E., und Horch R.E. (2016) [Concept for a National Implant Registry to Improve Patient Safety]. Handchir Mikrochir Plast Chir. 48: S. 320-329.
21. Prantl L., Gerken M., Zeman F., Leitzmann M., Koller M., Klinkhammer-Schalke M., Evert M., Kuehlmann B., und Biermann N. (2020) Incidence of Anaplastic Large Cell Lymphoma and Breast-Implant-Associated Lymphoma-An Analysis of a Certified Tumor Registry over 17 Years. J Clin Med. 9.
22. Radovan C. (1982) Breast reconstruction after mastectomy using the temporary expander. Plast Reconstr Surg. 69: S. 195-208.
23. Spear S.L. und Baker J.L., Jr. (1995) Classification of capsular contracture after prosthetic breast reconstruction. Plast Reconstr Surg. 96: S. 1119-23; discussion 1124.
24. Spear S.L., Schwartz J., Dayan J.H., und Clemens M.W. (2009) Outcome assessment of breast distortion following submuscular breast augmentation. Aesthetic Plast Surg. 33: S. 44-8.
25. von Fritschen U., Kremer T., Prantl L., und Fricke A. (2023) Breast Implant-Associated Tumors. Geburtshilfe Frauenheilkd. 83: S. 686-693.
26. von Fritschen U., Rakhorst H.A., Stark B., Ahern S., Prantl L., und Fricke A. (2023) The Mandatory German Breast Implant Registry Law: A Model for Sustainable Implant Registries. Aesthet Surg J. 43: S. NP858-NP865.
Videos
References